Related Research Articles

Natural killer cells, also known as NK cells or large granular lymphocytes (LGL), are a type of cytotoxic lymphocyte critical to the innate immune system. They belong to the rapidly expanding family of known innate lymphoid cells (ILC) and represent 5–20% of all circulating lymphocytes in humans. The role of NK cells is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK cells provide rapid responses to virus-infected cells, stressed cells, tumor cells, and other intracellular pathogens based on signals from several activating and inhibitory receptors. Most immune cells detect the antigen presented on major histocompatibility complex I (MHC-I) on infected cell surfaces, but NK cells can recognize and kill stressed cells in the absence of antibodies and MHC, allowing for a much faster immune reaction. They were named "natural killers" because of the notion that they do not require activation to kill cells that are missing "self" markers of MHC class I. This role is especially important because harmful cells that are missing MHC I markers cannot be detected and destroyed by other immune cells, such as T lymphocyte cells.
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies. Immunotherapy is under preliminary research for its potential to treat various forms of cancer.
A cancer vaccine, or oncovaccine, is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.

Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.

Fucose is a hexose deoxy sugar with the chemical formula C6H12O5. It is found on N-linked glycans on the mammalian, insect and plant cell surface. Fucose is the fundamental sub-unit of the seaweed polysaccharide fucoidan. The α(1→3) linked core of fucoidan is a suspected carbohydrate antigen for IgE-mediated allergy.
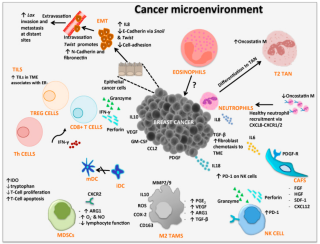
Cancer immunology (immuno-oncology) is an interdisciplinary branch of biology and a sub-discipline of immunology that is concerned with understanding the role of the immune system in the progression and development of cancer; the most well known application is cancer immunotherapy, which utilises the immune system as a treatment for cancer. Cancer immunosurveillance and immunoediting are based on protection against development of tumors in animal systems and (ii) identification of targets for immune recognition of human cancer.

Tumor-infiltrating lymphocytes (TIL) are white blood cells that have left the bloodstream and migrated towards a tumor. They include T cells and B cells and are part of the larger category of ‘tumor-infiltrating immune cells’ which consist of both mononuclear and polymorphonuclear immune cells, in variable proportions. Their abundance varies with tumor type and stage and in some cases relates to disease prognosis.

5′-nucleotidase (5′-NT), also known as ecto-5′-nucleotidase or CD73, is an enzyme that in humans is encoded by the NT5E gene. CD73 commonly serves to convert AMP to adenosine.

Programmed cell death protein 1(PD-1),. PD-1 is a protein encoded in humans by the PDCD1 gene. PD-1 is a cell surface receptor on T cells and B cells that has a role in regulating the immune system's response to the cells of the human body by down-regulating the immune system and promoting self-tolerance by suppressing T cell inflammatory activity. This prevents autoimmune diseases, but it can also prevent the immune system from killing cancer cells.

CD226, PTA1 or DNAM-1 is a ~65 kDa immunoglobulin-like transmembrane glycoprotein expressed on the surface of natural killer cells, NK T cell, B cells, dendritic cells, hematopoietic precursor cells, platelets, monocytes and T cells.

OX-2 membrane glycoprotein, also named CD200 is a human protein encoded by the CD200 gene. CD200 gene is in human located on chromosome 3 in proximity to genes encoding other B7 proteins CD80/CD86. In mice CD200 gene is on chromosome 16.
Vaccine therapy is a type of treatment that uses a substance or group of substances to stimulate the immune system to destroy a tumor or infectious microorganisms such as bacteria or viruses.
Adoptive cell transfer (ACT) is the transfer of cells into a patient. The cells may have originated from the patient or from another individual. The cells are most commonly derived from the immune system with the goal of improving immune functionality and characteristics. In autologous cancer immunotherapy, T cells are extracted from the patient, genetically modified and cultured in vitro and returned to the same patient. Comparatively, allogeneic therapies involve cells isolated and expanded from a donor separate from the patient receiving the cells.

The abscopal effect is a hypothesis in the treatment of metastatic cancer whereby shrinkage of untreated tumors occurs concurrently with shrinkage of tumors within the scope of the localized treatment. R.H. Mole proposed the term “abscopal” in 1953 to refer to effects of ionizing radiation “at a distance from the irradiated volume but within the same organism.”
Myeloid-derived suppressor cells (MDSC) are a heterogeneous group of immune cells from the myeloid lineage.
Cancer/testis (CT) antigens are a group of proteins united by their importance in development and in cancer immunotherapy. In general, expression of these proteins is restricted to male germ cells in the adult animal. However, in cancer these developmental antigens are often re-expressed and can serve as a locus of immune activation. Thus, they are often classified as tumor antigens. The expression of CT antigens in various malignancies is heterogeneous and often correlates with tumor progression. CT antigens have been described in melanoma, liver cancer, lung cancer, bladder cancer, and pediatric tumors such as neuroblastoma. Gametogenesis offers an important role for many of these antigens in the differentiation, migration, and cell division of primordial germ cells, spermatogonia spermatocytes and spermatids. Because of their tumor-restricted expression and strong in vivo immunogenicity, CT antigens are identified as ideal targets for tumor specific immunotherapeutic approaches and prompted the development of several clinical trials of CT antigens-based vaccine therapy. CT antigens have been found to have at least 70 families so far, including about 140 members, most of which are expressed during spermatogenesis. Their expression are mainly regulated by epigenetic events, specifically, DNA methylation.
The Immunologic Constant of Rejection (ICR), is a notion introduced by biologists to group a shared set of genes expressed in tissue destructive-pathogenic conditions like cancer and infection, along a diverse set of physiological circumstances of tissue damage or organ failure, including autoimmune disease or allograft rejection. The identification of shared mechanisms and phenotypes by distinct immune pathologies, marked as a hallmarks or biomarkers, aids in the identification of novel treatment options, without necessarily assessing patients phenomenologies individually.
Checkpoint inhibitor therapy is a form of cancer immunotherapy. The therapy targets immune checkpoints, key regulators of the immune system that when stimulated can dampen the immune response to an immunologic stimulus. Some cancers can protect themselves from attack by stimulating immune checkpoint targets. Checkpoint therapy can block inhibitory checkpoints, restoring immune system function. The first anti-cancer drug targeting an immune checkpoint was ipilimumab, a CTLA4 blocker approved in the United States in 2011.
Individualized cancer immunotherapy, also referred to as individualized immuno-oncology, is a novel concept for therapeutic cancer vaccines that are truly personalized to a single individual.

Cellular adoptive immunotherapy is a type of immunotherapy. Immune cells such as T-cells are usually isolated from patients for expansion or engineering purposes and reinfused back into patients to fight diseases using their own immune system. A major application of cellular adoptive therapy is cancer treatment, as the immune system plays a vital role in the development and growth of cancer. The primary types of cellular adoptive immunotherapies are T cell therapies. Other therapies include CAR-T therapy, CAR-NK therapy, macrophage-based immunotherapy and dendritic cell therapy.
References
- 1 2 "Fucosylation in prokaryotes and eukaryotes". academic.oup.com. Retrieved 2 November 2023.
- ↑ Ma, B.; Simala-Grant, J. L.; Taylor, D. E. (2006). "Fucosylation in prokaryotes and eukaryotes". Glycobiology. 16 (12): 158R–184R. doi: 10.1093/glycob/cwl040 . PMID 16973733.
- ↑ Miyoshi, E.; Moriwaki, K.; Nakagawa, T. (2007). "Biological Function of Fucosylation in Cancer Biology". Journal of Biochemistry. 143 (6): 725–729. doi:10.1093/jb/mvn011. PMID 18218651.
- ↑ Miyoshi, Eiji (2008). "Fucosylation and Cancer". Experimental Glycoscience. Springer. pp. 235–7. doi:10.1007/978-4-431-77922-3_57. ISBN 978-4-431-77921-6.
- ↑ Nakagawa, T.; Uozumi, N; Nakano, M; Mizuno-Horikawa, Y; Okuyama, N; Taguchi, T; Gu, J; Kondo, A; et al. (2006). "Fucosylation of N-Glycans Regulates the Secretion of Hepatic Glycoproteins into Bile Ducts". Journal of Biological Chemistry. 281 (40): 29797–29806. doi: 10.1074/jbc.M605697200 . PMID 16899455.
- ↑ Moriwaki, Kenta (2010). "Fucosylation and gastrointestinal cancer". World Journal of Hepatology. 2 (4): 151–161. doi: 10.4254/wjh.v2.i4.151 . PMC 2999278 . PMID 21160988.
- ↑ Li J, Hsu HC, Mountz JD, Allen JG (May 2018). "Unmasking Fucosylation: from Cell Adhesion to Immune System Regulation and Diseases". Cell Chem Biol. 25 (5): 499–512. doi: 10.1016/j.chembiol.2018.02.005 . PMID 29526711.
- 1 2 Lester, Daniel K.; Burton, Chase; Gardner, Alycia; Innamarato, Patrick; Kodumudi, Krithika; Liu, Qian; Adhikari, Emma; Ming, Qianqian; Williamson, Daniel B.; Frederick, Dennie T.; Sharova, Tatyana; White, Michael G.; Markowitz, Joseph; Cao, Biwei; Nguyen, Jonathan (February 2023). "Fucosylation of HLA-DRB1 regulates CD4+ T cell-mediated anti-melanoma immunity and enhances immunotherapy efficacy". Nature Cancer. 4 (2): 222–239. doi:10.1038/s43018-022-00506-7. ISSN 2662-1347. PMC 9970875 . PMID 36690875.
- ↑ Pickard, Joseph M.; Maurice, Corinne F.; Kinnebrew, Melissa A.; Abt, Michael C.; Schenten, Dominik; Golovkina, Tatyana V.; Bogatyrev, Said R.; Ismagilov, Rustem F.; Pamer, Eric G.; Turnbaugh, Peter J.; Chervonsky, Alexander V. (October 2014). "Rapid fucosylation of intestinal epithelium sustains host–commensal symbiosis in sickness". Nature. 514 (7524): 638–641. Bibcode:2014Natur.514..638P. doi:10.1038/nature13823. PMC 4214913 . PMID 25274297.