
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G proteins. They pass through the cell membrane seven times in form of six loops of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors. Ligands can bind either to the extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists, although a spontaneous auto-activation of an empty receptor has also been observed.

A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted using denaturing agents.

Saccharomyces cerevisiae is a species of yeast. The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have been originally isolated from the skin of grapes. It is one of the most intensively studied eukaryotic model organisms in molecular and cell biology, much like Escherichia coli as the model bacterium. It is the microorganism behind the most common type of fermentation. S. cerevisiae cells are round to ovoid, 5–10 μm in diameter. It reproduces by budding.
A signal peptide is a short peptide present at the N-terminus of most newly synthesized proteins that are destined toward the secretory pathway. These proteins include those that reside either inside certain organelles, secreted from the cell, or inserted into most cellular membranes. Although most type I membrane-bound proteins have signal peptides, the majority of type II and multi-spanning membrane-bound proteins are targeted to the secretory pathway by their first transmembrane domain, which biochemically resembles a signal sequence except that it is not cleaved. They are a kind of target peptide.

Protein–protein interactions (PPIs) are physical contacts of high specificity established between two or more protein molecules as a result of biochemical events steered by interactions that include electrostatic forces, hydrogen bonding and the hydrophobic effect. Many are physical contacts with molecular associations between chains that occur in a cell or in a living organism in a specific biomolecular context.
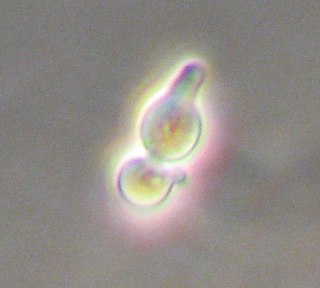
The yeast Saccharomyces cerevisiae is a simple single-celled eukaryote with both a diploid and haploid mode of existence. The mating of yeast only occurs between haploids, which can be either the a or α (alpha) mating type and thus display simple sexual differentiation. Mating type is determined by a single locus, MAT, which in turn governs the sexual behaviour of both haploid and diploid cells. Through a form of genetic recombination, haploid yeast can switch mating type as often as every cell cycle.

A fungal prion is a prion that infects hosts which are fungi. Fungal prions are naturally occurring proteins that can switch between multiple, structurally distinct conformations, at least one of which is self-propagating and transmissible to other prions. This transmission of protein state represents an epigenetic phenomenon where information is encoded in the protein structure itself, instead of in nucleic acids. Several prion-forming proteins have been identified in fungi, primarily in the yeast Saccharomyces cerevisiae. These fungal prions are generally considered benign, and in some cases even confer a selectable advantage to the organism.
In biology, cell signaling or cell communication is the ability of a cell to receive, process, and transmit signals with its environment and with itself. Cell signaling is a fundamental property of all cellular life in prokaryotes and eukaryotes. Signals that originate from outside a cell can be physical agents like mechanical pressure, voltage, temperature, light, or chemical signals. Cell signaling can occur over short or long distances, and as a result can be classified as autocrine, juxtacrine, intracrine, paracrine, or endocrine. Signaling molecules can be synthesized from various biosynthetic pathways and released through passive or active transports, or even from cell damage.

Transforming growth factor alpha (TGF-α) is a protein that in humans is encoded by the TGFA gene. As a member of the epidermal growth factor (EGF) family, TGF-α is a mitogenic polypeptide. The protein becomes activated when binding to receptors capable of protein kinase activity for cellular signaling.

The granulocyte-macrophage colony-stimulating factor receptor also known as CD116, is a receptor for granulocyte-macrophage colony-stimulating factor, which stimulates the production of white blood cells. In contrast to M-CSF and G-CSF which are lineage specific, GM-CSF and its receptor play a role in earlier stages of development. The receptor is primarily located on neutrophils, eosinophils and monocytes/macrophages, it is also on CD34+ progenitor cells (myeloblasts) and precursors for erythroid and megakaryocytic lineages, but only in the beginning of their development.
Regulator of G protein signaling 4 also known as RGP4 is a protein that in humans is encoded by the RGS4 gene. RGP4 regulates G protein signaling.

Corticotropin-releasing hormone receptor 2 (CRHR2) is a protein, also known by the IUPHAR-recommended name CRF2, that is encoded by the CRHR2 gene and occurs on the surfaces of some mammalian cells. CRF2 receptors are type 2 G protein-coupled receptors for corticotropin-releasing hormone (CRH) that are resident in the plasma membranes of hormone-sensitive cells. CRH, a peptide of 41 amino acids synthesized in the hypothalamus, is the principal neuroregulator of the hypothalamic-pituitary-adrenal axis, signaling via guanine nucleotide-binding proteins (G proteins) and downstream effectors such as adenylate cyclase. The CRF2 receptor is a multi-pass membrane protein with a transmembrane domain composed of seven helices arranged in a V-shape. CRF2 receptors are activated by two structurally similar peptides, urocortin II, and urocortin III, as well as CRH.
T-cell receptor alpha locus is a protein that in humans is encoded by the TRA gene, also known as TCRA or TRA@. It contributes the alpha chain to the larger TCR protein.
A killer yeast is a yeast, such as Saccharomyces cerevisiae, which is able to secrete one of a number of toxic proteins which are lethal to susceptible cells. These "killer toxins" are polypeptides that kill sensitive cells of the same or related species, often functioning by creating pores in target cell membranes. These yeast cells are immune to the toxic effects of the protein due to an intrinsic immunity. Killer yeast strains can be a problem in commercial processing because they can kill desirable strains. The killer yeast system was first described in 1963. Study of killer toxins helped to better understand the secretion pathway of yeast, which is similar to those of more complex eukaryotes. It also can be used in treatment of some diseases, mainly those caused by fungi.

Pho4 is a protein with a basic helix-loop-helix (bHLH) transcription factor. It is found in S. cerevisiae and other yeasts. It functions as a transcription factor to regulate phosphate responsive genes located in yeast cells. The Pho4 protein homodimer is able to do this by binding to DNA sequences containing the bHLH binding site 5'-CACGTG-3'. This sequence is found in the promoters of genes up-regulated in response to phosphate availability such as the PHO5 gene.
Amino acid permeases are membrane permeases involved in the transport of amino acids into the cell. A number of such proteins have been found to be evolutionary related. These proteins contain 12 transmembrane segments.
SNED1 is an extracellular matrix (ECM) protein expressed at low levels in a wide range of tissues. The gene encoding SNED1 is located in the human chromosome 2 at locus q37.3. The corresponding mRNA isolated from the spleen and is 6834bp in length, and the corresponding protein is 1413 amino-acid long. The mouse ortholog of SNED1 was cloned in 2004 from the embryonic kidney by Leimester et al. SNED1 present domains characteristic of ECM proteins, including an amino-terminal NIDO domain, several calcium binding EGF-like domains (EGF_CA), a Sushi domain also known as complement control protein (CCP) domain, and three type III fibronectin (FN3) domains in the carboxy-terminal region.
The Gal4 transcription factor is a positive regulator of gene expression of galactose-induced genes. This protein represents a large fungal family of transcription factors, Gal4 family, which includes over 50 members in the yeast Saccharomyces cerevisiae e.g. Oaf1, Pip2, Pdr1, Pdr3, Leu3.
Membranome database provides structural and functional information about more than 6000 single-pass (bitopic) transmembrane proteins from Homo sapiens, Arabidopsis thaliana, Dictyostelium discoideum, Saccharomyces cerevisiae, Escherichia coli and Methanocaldococcus jannaschii. Bitopic membrane proteins consist of a single transmembrane alpha-helix connecting water-soluble domains of the protein situated at the opposite sides of a biological membrane. These proteins are frequently involved in the signal transduction and communication between cells in multicellular organisms.
Shuguang Zhang is an American biochemist. He is at the MIT Media Lab's Laboratory for Molecular Architecture. Shuguang Zhang's research focuses on designs of biological molecules, particularly proteins and peptides. He has published over 170 scientific papers, which have cumulatively been cited over 35,000 times with an h-index of 88. On the “Updated science-wide author databases of standardizes citation indicators”, he is ranked 18th worldwide in the field of Biomedical Engineering. Zhang is also a co-founder and board member of Molecular Frontiers Foundation, which organizes annual Molecular Frontiers Symposia in Sweden and around the world. The selected winners are awarded Molecular Frontiers Inquiry Prize.











