Related Research Articles

Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen or moisture slowly to form a black coating. Gadolinium below its Curie point of 20 °C (68 °F) is ferromagnetic, with an attraction to a magnetic field higher than that of nickel. Above this temperature it is the most paramagnetic element. It is found in nature only in an oxidized form. When separated, it usually has impurities of the other rare-earths because of their similar chemical properties.

Magnetic resonance imaging (MRI) is a medical imaging technique used in radiology to form pictures of the anatomy and the physiological processes of the body. MRI scanners use strong magnetic fields, magnetic field gradients, and radio waves to generate images of the organs in the body. MRI does not involve X-rays or the use of ionizing radiation, which distinguishes it from CT or CAT scans and PET scans. Magnetic resonance imaging is a medical application of nuclear magnetic resonance (NMR). NMR can also be used for imaging in other NMR applications such as NMR spectroscopy.
Superparamagnetism is a form of magnetism which appears in small ferromagnetic or ferrimagnetic nanoparticles. In sufficiently small nanoparticles, magnetization can randomly flip direction under the influence of temperature. The typical time between two flips is called the Néel relaxation time. In the absence of an external magnetic field, when the time used to measure the magnetization of the nanoparticles is much longer than the Néel relaxation time, their magnetization appears to be in average zero; they are said to be in the superparamagnetic state. In this state, an external magnetic field is able to magnetize the nanoparticles, similarly to a paramagnet. However, their magnetic susceptibility is much larger than that of paramagnets.

Nafion is a brand name for a sulfonated tetrafluoroethylene based fluoropolymer-copolymer discovered in the late 1960s by Walther Grot of DuPont. Nafion is a brand of the Chemours company. It is the first of a class of synthetic polymers with ionic properties that are called ionomers. Nafion's unique ionic properties are a result of incorporating perfluorovinyl ether groups terminated with sulfonate groups onto a tetrafluoroethylene (PTFE) backbone. Nafion has received a considerable amount of attention as a proton conductor for proton exchange membrane (PEM) fuel cells because of its excellent thermal and mechanical stability.

Iron(II,III) oxide is the chemical compound with formula Fe3O4. It occurs in nature as the mineral magnetite. It is one of a number of iron oxides, the others being iron(II) oxide (FeO), which is rare, and iron(III) oxide (Fe2O3) also known as hematite. It contains both Fe2+ and Fe3+ ions and is sometimes formulated as FeO ∙ Fe2O3. This iron oxide is encountered in the laboratory as a black powder. It exhibits permanent magnetism and is ferrimagnetic, but is sometimes incorrectly described as ferromagnetic. Its most extensive use is as a black pigment. For this purpose, it is synthesised rather than being extracted from the naturally occurring mineral as the particle size and shape can be varied by the method of production.
Aqueous biphasic systems (ABS) or aqueous two-phase systems (ATPS) are clean alternatives for traditional organic-water solvent extraction systems.
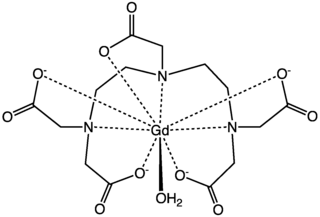
Gadopentetic acid is one of the trade names for a gadolinium-based MRI contrast agent, usually administered as a salt of a complex of gadolinium with DTPA (diethylenetriaminepentacetate) with the chemical formula A2[Gd(DTPA)(H2O)]; when cation A is the protonated form of the amino sugar meglumine the salt goes under the name "gadopentetate dimeglumine". It was described in 1981 by Hanns-Joachim Weinmann and colleagues and introduced as the first MRI contrast agent in 1987 by the Schering AG. It is used to assist imaging of blood vessels and of inflamed or diseased tissue where the blood vessels become "leaky". It is often used when viewing intracranial lesions with abnormal vascularity or abnormalities in the blood–brain barrier. It is usually injected intravenously. Gd-DTPA is classed as an acyclic, ionic gadolinium contrast medium. Its paramagnetic property reduces the T1 relaxation time (and to some extent the T2 and T2* relaxation times) in NMR, which is the source of its clinical utility.
Nanochemistry is the combination of chemistry and nanoscience. Nanochemistry is associated with synthesis of building blocks which are dependent on size, surface, shape and defect properties. Nanochemistry is being used in chemical, materials and physical, science as well as engineering, biological and medical applications. Nanochemistry and other nanoscience fields have the same core concepts but the usages of those concepts are different.

Gadolinium(III) oxide (archaically gadolinia) is an inorganic compound with the formula Gd2O3. It is one of the most commonly available forms of the rare-earth element gadolinium, derivatives of which are potential contrast agents for magnetic resonance imaging.
Nanocomposite is a multiphase solid material where one of the phases has one, two or three dimensions of less than 100 nanometers (nm) or structures having nano-scale repeat distances between the different phases that make up the material.
Nuclear magnetic resonance (NMR) in the geomagnetic field is conventionally referred to as Earth's field NMR (EFNMR). EFNMR is a special case of low field NMR.
MRI contrast agents are contrast agents used to improve the visibility of internal body structures in magnetic resonance imaging (MRI). The most commonly used compounds for contrast enhancement are gadolinium-based. Such MRI contrast agents shorten the relaxation times of nuclei within body tissues following oral or intravenous administration.
Molecular imaging is broadly defined as the visualization of molecular and cellular processes on either a macro- or microscopic level. Because of its high spatial resolution and ability to noninvasively visualize internal organs, magnetic resonance (MR) imaging is widely believed to be an ideal platform for in vivo molecular imaging. For this reason, MR contrast agents that can detect molecular events are an active field of research. One group of compounds that has shown particular promise is enzyme-activated MR contrast agents.
Magnetic nanoparticles are a class of nanoparticle that can be manipulated using magnetic fields. Such particles commonly consist of two components, a magnetic material, often iron, nickel and cobalt, and a chemical component that has functionality. While nanoparticles are smaller than 1 micrometer in diameter, the larger microbeads are 0.5–500 micrometer in diameter. Magnetic nanoparticle clusters that are composed of a number of individual magnetic nanoparticles are known as magnetic nanobeads with a diameter of 50–200 nanometers. Magnetic nanoparticle clusters are a basis for their further magnetic assembly into magnetic nanochains. The magnetic nanoparticles have been the focus of much research recently because they possess attractive properties which could see potential use in catalysis including nanomaterial-based catalysts, biomedicine and tissue specific targeting, magnetically tunable colloidal photonic crystals, microfluidics, magnetic resonance imaging, magnetic particle imaging, data storage, environmental remediation, nanofluids,, optical filters, defect sensor, magnetic cooling and cation sensors.
In polymer chemistry, in situ polymerization is a preparation method that occurs "in the polymerization mixture" and is used to develop polymer nanocomposites from nanoparticles. There are numerous unstable oligomers (molecules) which must be synthesized in situ for use in various processes. The in situ polymerization process consists of an initiation step followed by a series of polymerization steps, which results in the formation of a hybrid between polymer molecules and nanoparticles. Nanoparticles are initially spread out in a liquid monomer or a precursor of relatively low molecular weight. Upon the formation of a homogenous mixture, initiation of the polymerization reaction is carried out by addition of an adequate initiator, which is exposed to a source of heat, radiation, etc. After the polymerization mechanism is completed, a nanocomposite is produced, which consists of polymer molecules bound to nanoparticles.

Nuclear magnetic resonance (NMR) is a method of physical observation in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics, crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI).
Iron oxide nanoparticles are iron oxide particles with diameters between about 1 and 100 nanometers. The two main forms are magnetite (Fe3O4) and its oxidized form maghemite (γ-Fe2O3). They have attracted extensive interest due to their superparamagnetic properties and their potential applications in many fields (although Co and Ni are also highly magnetic materials, they are toxic and easily oxidized).
The behavior of quantum dots (QDs) in solution and their interaction with other surfaces is of great importance to biological and industrial applications, such as optical displays, animal tagging, anti-counterfeiting dyes and paints, chemical sensing, and fluorescent tagging. However, unmodified quantum dots tend to be hydrophobic, which precludes their use in stable, water-based colloids. Furthermore, because the ratio of surface area to volume in a quantum dot is much higher than for larger particles, the thermodynamic free energy associated with dangling bonds on the surface is sufficient to impede the quantum confinement of excitons. Once solubilized by encapsulation in either a hydrophobic interior micelle or a hydrophilic exterior micelle, the QDs can be successfully introduced into an aqueous medium, in which they form an extended hydrogel network. In this form, quantum dots can be utilized in several applications that benefit from their unique properties, such as medical imaging and thermal destruction of malignant cancers.
Nanocomposite hydrogels are nanomaterial-filled, hydrated, polymeric networks that exhibit higher elasticity and strength relative to traditionally made hydrogels. A range of natural and synthetic polymers are used to design nanocomposite network. By controlling the interactions between nanoparticles and polymer chains, a range of physical, chemical, and biological properties can be engineered. The combination of organic (polymer) and inorganic (clay) structure gives these hydrogels improved physical, chemical, electrical, biological, and swelling/de-swelling properties that cannot be achieved by either material alone. Inspired by flexible biological tissues, researchers incorporate carbon-based, polymeric, ceramic and/or metallic nanomaterials to give these hydrogels superior characteristics like optical properties and stimulus-sensitivity which can potentially be very helpful to medical and mechanical fields.
Magnetoelastic filaments are one-dimensional composite structures that exhibit both magnetic and elastic properties. Interest in these materials tends to focus on the ability to precisely control mechanical events using an external magnetic field. Like piezoelectricity materials, they can be used as actuators, but do not need to be physically connected to a power source. The conformations adopted by magnetoelastic filaments are dictated by the competition between its elastic and magnetic properties.
References
- 1 2 Sitharaman B, Kissell KR, Hartman KB, Tran LA, Baikalov A, Rusakova I, Sun Y, Khant HA, Ludtke SJ, Chiu W, Laus S, Tóth E, Helm L, Merbach AE, Wilson LJ (2005). "Superparamagnetic gadonanotubes are high-performance MRI contrast agents" (PDF). Chemical Communications (31): 3915–7. doi:10.1039/b504435a. PMID 16075070. supplementary information
- 1 2 3 4 Chan, Warren C. W. (2007). Bio-Applications of Nanoparticles. Springer. pp. 79–. ISBN 978-0-387-76713-0.
- 1 2 Wilson, Lon J.; Muthupillai, Raja; Perin, Emerson C.; Willerson, James T.; Mowlazadeh-Haghighi, Saghar; da Graça Cabreira-Hansen, Maria; Ajala, Afis; Zaibaq, Nicholas G.; Hernández-Rivera, Mayra (2018). "A New High-Performance Gadonanotube-Polymer Hybrid Material for Stem Cell Labeling and Tracking by MRI". Contrast Media & Molecular Imaging. 2018: 2853736. doi:10.1155/2018/2853736. PMC 6079544 . PMID 30116161.