
Retroviral integrase (IN) is an enzyme produced by a retrovirus that integrates—forms covalent links between—its genetic information into that of the host cell it infects. Retroviral INs are not to be confused with phage integrases (recombinases) used in biotechnology, such as λ phage integrase, as discussed in site-specific recombination.

Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Unlike most antibiotics, antiviral drugs do not destroy their target pathogen; instead they inhibit its development.
Reverse-transcriptase inhibitors (RTIs) are a class of antiretroviral drugs used to treat HIV infection or AIDS, and in some cases hepatitis B. RTIs inhibit activity of reverse transcriptase, a viral DNA polymerase that is required for replication of HIV and other retroviruses.

Eucalyptus globoidea, commonly known as the white stringybark, is a tree that is endemic to near-coastal areas of south-eastern Australia. It has rough, stringy bark, often furrowed on the trunk, glossy, lance-shaped to egg-shaped, often curved leaves, oval to spindle-shaped green to yellowish flower buds, white flowers and small, more or less spherical to hemispherical fruit.
Integrase inhibitors (INIs) are a class of antiretroviral drug designed to block the action of integrase, a viral enzyme that inserts the viral genome into the DNA of the host cell. Since integration is a vital step in retroviral replication, blocking it can halt further spread of the virus. Integrase inhibitors were initially developed for the treatment of HIV infection, but have been applied to other retroviruses. The class of integrase inhibitors called integrase strand transfer inhibitors (INSTIs) are in established medical use. Other classes, such as integrase binding inhibitors (INBIs), are still experimental.

A resistance mutation is a mutation in a virus gene that allows the virus to become resistant to treatment with a particular antiviral drug. The term was first used in the management of HIV, the first virus in which genome sequencing was routinely used to look for drug resistance. At the time of infection, a virus will infect and begin to replicate within a preliminary cell. As subsequent cells are infected, random mutations will occur in the viral genome. When these mutations begin to accumulate, antiviral methods will kill the wild type strain, but will not be able to kill one or many mutated forms of the original virus. At this point a resistance mutation has occurred because the new strain of virus is now resistant to the antiviral treatment that would have killed the original virus. Resistance mutations are evident and widely studied in HIV due to its high rate of mutation and prevalence in the general population. Resistance mutation is now studied in bacteriology and parasitology.

HIV-1 protease (PR) is a retroviral aspartyl protease (retropepsin), an enzyme involved with peptide bond hydrolysis in retroviruses, that is essential for the life-cycle of HIV, the retrovirus that causes AIDS. HIV protease cleaves newly synthesized polyproteins at nine cleavage sites to create the mature protein components of an HIV virion, the infectious form of a virus outside of the host cell. Without effective HIV protease, HIV virions remain uninfectious.
A portmanteau inhibitor is a drug that is a combination of two drug molecules, each of which is itself a type of inhibitor. The term was coined in 2007 by University of Minnesota researchers who designed and synthesized a combination HIV reverse transcriptase inhibitor and an integrase inhibitor, and was further used in 2011 by a team of researchers combining an integrase inhibitor with a CCR5 entry inhibitor.

Elvitegravir (EVG) is an integrase inhibitor used to treat HIV infection. It was developed by the pharmaceutical company Gilead Sciences, which licensed EVG from Japan Tobacco in March 2008. The drug gained approval by the U.S. Food and Drug Administration on August 27, 2012 for use in adult patients starting HIV treatment for the first time as part of the fixed dose combination known as Stribild. On September 24, 2014 the FDA approved Elvitegravir as a single pill formulation under the trade name Vitekta. On November 5, 2015 the FDA approved the drug for use in patients affected with HIV-1 as a part of a second fixed dose combination pill known as Genvoya.

AIDS is caused by the human immunodeficiency virus (HIV). Individuals with HIV have what is referred to as a "HIV infection". When infected semen, vaginal secretions, or blood come in contact with the mucous membranes or broken skin of an uninfected person, HIV may be transferred to the uninfected person, causing another infection. Additionally, HIV can also be passed from infected pregnant women to their uninfected baby during pregnancy and/or delivery, or via breastfeeding. As a result of HIV infection, a portion of these individuals will progress and go on to develop clinically significant AIDS.

A depside is a type of polyphenolic compound composed of two or more monocyclic aromatic units linked by an ester bond. Depsides are most often found in lichens, but have also been isolated from higher plants, including species of the Ericaceae, Lamiaceae, Papaveraceae and Myrtaceae.

MK-2048 is the Merck & Co. designation for a molecule in its pre-clinical drug discovery that is an integrase inhibitor-class of agent intended to be used against HIV infection. It is a second generation integrase design thought to be superior to the first available integrase inhibitor, raltegravir, in that "MK-2048 has a dissociation half-life of 32 hours on wild-type integrase—more than four times that of raltegravir", and its dissociation half-life against the important HIV integrase mutant N155H was on the same order of magnitude as that of raltegravir against wild-type virus, leading the Merck presenter to suggest the possibility of "reduced susceptibility to resistance mutations" for the second generation drug. MK-2048 is being investigated for use as part of a pre-exposure prophylaxis (PrEP) approach to the treatment of HIV infection. At the time of these reports, there was no indication of the time by which "MK-2048, or related compounds, [would] be ready for clinical trials".

Integrasone is a polyketide natural product, isolated from an unknown fungus, that has been shown to inhibit the HIV-1 integrase enzyme.
Discovery and development of nucleoside and nucleotide reverse-transcriptase inhibitors began in the 1980s when the AIDS epidemic hit Western societies. NRTIs inhibit the reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of the human immunodeficiency virus (HIV). The first NRTI was zidovudine, approved by the U.S. Food and Drug Administration (FDA) in 1987, which was the first step towards treatment of HIV. Six NRTI agents and one NtRTI have followed. The NRTIs and the NtRTI are analogues of endogenous 2´-deoxy-nucleoside and nucleotide. Drug-resistant viruses are an inevitable consequence of prolonged exposure of HIV-1 to anti-HIV drugs.
The first human immunodeficiency virus (HIV) case was reported in the United States in the early 1980s. Many drugs have been discovered to treat the disease but mutations in the virus and resistance to the drugs make development difficult. Integrase is a viral enzyme that integrates retroviral DNA into the host cell genome. Integrase inhibitors are a new class of drugs used in the treatment of HIV. The first integrase inhibitor, raltegravir, was approved in 2007 and other drugs were in clinical trials in 2011.
The ChemDB HIV, Opportunistic Infection and Tuberculosis Therapeutics Database is a publicly available tool developed by the National Institute of Allergy and Infectious Diseases to compile preclinical data on small molecules with potential therapeutic action against HIV/AIDS and related opportunistic infections.
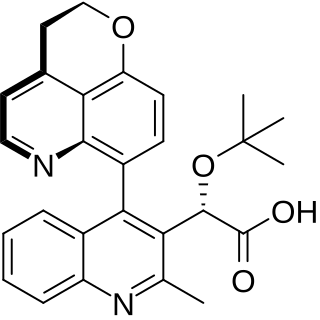
BI 224436 was an investigational new drug under development for the treatment of HIV infection. BI 224436 is the first non-catalytic site integrase inhibitor (NCINI). It inhibits HIV replication via binding to a conserved allosteric pocket of the HIV integrase enzyme. This makes the drug distinct in its mechanism of action compared to raltegravir and elvitegravir, which bind at the catalytic site. In October 2011, Gilead Sciences purchased exclusive rights to develop BI 224436 and several related compounds under investigation in Boehringer Ingelheim’s noncatalytic site integrase inhibitor program.

Abacavir/dolutegravir/lamivudine, sold under the brand name Triumeq among others, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. It is a combination of three medications with different and complementary mechanisms of action: abacavir, dolutegravir and lamivudine.
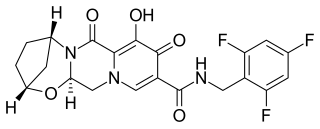
Bictegravir is a second-generation integrase inhibitor (INSTI) class that was structurally derived from an earlier compound dolutegravir by scientists at Gilead Sciences; in vitro and clinical results were presented by Gilead in the summer of 2016. In 2016, bictegravir was in a Phase 3 trial as part of a single tablet regimen in combination with tenofovir alafenamide (TAF) and emtricitabine (FTC) for the treatment of HIV-1 infection and the combination drug bictegravir/emtricitabine/tenofovir alafenamide (Biktarvy) was approved for use in the United States in 2018.
Daria J Hazuda is a biochemist known for discovering the first HIV Integrase Strand Transfer Inhibitors (InSTIs) and leading the development of the first HIV integrase inhibitor to gain FDA approval, Isentress (raltegravir). Her lab also determined these inhibitors' mechanism of action and ways the virus could develop resistance to them. Hazuda has also performed extensive research and led the development of antivirals for Hepatitis C Virus (HCV) including Elbasvir and Grazoprevir. She currently serves as Vice President for Infectious Diseases Discovery for Merck and Chief Scientific Officer of their MRL Cambridge Exploratory Science Center.













