A glycosidic bond or glycosidic linkage is a type of ether bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.

Gilbert syndrome (GS) is a syndrome in which the liver of affected individuals processes bilirubin more slowly than the majority. Many people never have symptoms. Occasionally jaundice may occur.

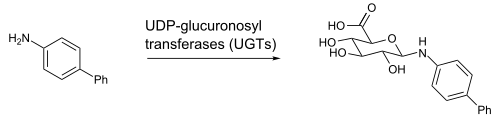
A glucuronide, also known as glucuronoside, is any substance produced by linking glucuronic acid to another substance via a glycosidic bond. The glucuronides belong to the glycosides.

Enterohepatic circulation is the circulation of biliary acids, bilirubin, drugs or other substances from the liver to the bile, followed by entry into the small intestine, absorption by the enterocyte and transport back to the liver. Enterohepatic circulation is an especially important concept in the field of toxicology as many lipophilic xenobiotics undergo this process causing repeated liver damage.

Glucuronic acid is a uronic acid that was first isolated from urine. It is found in many gums such as gum arabic, xanthan, and kombucha tea and is important for the metabolism of microorganisms, plants and animals.

Crigler–Najjar syndrome is a rare inherited disorder affecting the metabolism of bilirubin, a chemical formed from the breakdown of the heme in red blood cells. The disorder results in a form of nonhemolytic jaundice, which results in high levels of unconjugated bilirubin and often leads to brain damage in infants. The disorder is inherited in an autosomal recessive manner. The annual incidence is estimated at 1 in 1,000,000.

Uridine 5'-diphospho-glucuronosyltransferase is a microsomal glycosyltransferase that catalyzes the transfer of the glucuronic acid component of UDP-glucuronic acid to a small hydrophobic molecule. This is a glucuronidation reaction.

UDP-glucuronosyltransferase 1-1 also known as UGT-1A is an enzyme that in humans is encoded by the UGT1A1 gene.

UGT2B7 (UDP-Glucuronosyltransferase-2B7) is a phase II metabolism isoenzyme found to be active in the liver, kidneys, epithelial cells of the lower gastrointestinal tract and also has been reported in the brain. In humans, UDP-Glucuronosyltransferase-2B7 is encoded by the UGT2B7 gene.

Uridine diphosphate glucose is a nucleotide sugar. It is involved in glycosyltransferase reactions in metabolism.

UDP-glucuronosyltransferase 1-6 is an enzyme that in humans is encoded by the UGT1A6 gene.

UDP-glucuronosyltransferase 1-10 is an enzyme that in humans is encoded by the UGT1A10 gene.

UDP-glucuronosyltransferase 2B15 is an enzyme that in humans is encoded by the UGT2B15 gene.

UDP-glucuronosyltransferase 1-3 is an enzyme that in humans is encoded by the UGT1A3 gene.

UDP-glucuronosyltransferase 1-4 is an enzyme that in humans is encoded by the UGT1A4 gene.

UDP glucuronosyltransferase 2 family, polypeptide B4, also known as UGT2B4, is an enzyme that in humans is encoded by the UGT2B4 gene.

UDP-glucuronosyltransferase 2B17 is an enzyme that in humans is encoded by the UGT2B17 gene.

Hyodeoxycholic acid, also known as 3α,6α-Dihydroxy-5β-cholan-24-oic acid or HDCA, is a secondary bile acid, one of the metabolic byproducts of intestinal bacteria. It differs from deoxycholic acid in that the 6α-hydroxyl is in the 12 position in the former. The 6α-hydroxyl group makes HDCA a hydrophilic acid, a property it shares with hyocholic acid. HDCA is present in mammalian species in different proportions. It is the main acid constituent of hog bile, and for this reason it was used industrially as precursor for steroid synthesis before total synthesis became practical.

UDP-glucuronosyltransferase 1-9 is an enzyme that in humans is encoded by the UGT1A9 gene.

Bilirubin glucuronide is a water-soluble reaction intermediate over the process of conjugation of indirect bilirubin. Bilirubin glucuronide itself belongs to the category of conjugated bilirubin along with bilirubin di-glucuronide. However, only the latter one is primarily excreted into the bile in the normal setting.