Related Research Articles

Friedrich Wöhler FRS(For) HonFRSE was a German chemist known for his work in both organic and inorganic chemistry, being the first to isolate the chemical elements beryllium and yttrium in pure metallic form. He was the first to prepare several inorganic compounds, including silane and silicon nitride.
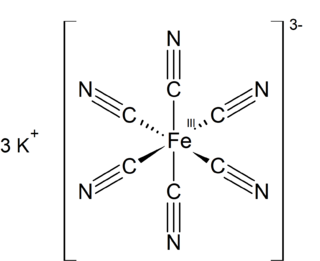
Potassium ferricyanide is the chemical compound with the formula K3[Fe(CN)6]. This bright red salt contains the octahedrally coordinated [Fe(CN)6]3− ion. It is soluble in water and its solution shows some green-yellow fluorescence. It was discovered in 1822 by Leopold Gmelin.

Leopold Gmelin was a German chemist. Gmelin was a professor at the University of Heidelberg. He worked on the red prussiate and created Gmelin's test, and wrote his Handbook of Chemistry, which over successive editions became a standard reference work still in use.

Barium hydroxide is a chemical compound with the chemical formula Ba(OH)2. The monohydrate (x = 1), known as baryta or baryta-water, is one of the principal compounds of barium. This white granular monohydrate is the usual commercial form.
Cuprates are a class of compounds that contain copper (Cu) atom(s) in an anion. They can be broadly categorized into two main types:

Samarium(II) iodide is an inorganic compound with the formula SmI2. When employed as a solution for organic synthesis, it is known as Kagan's reagent. SmI2 is a green solid and solutions are green as well. It is a strong one-electron reducing agent that is used in organic synthesis.

Hydrogen bromide is the inorganic compound with the formula HBr. It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temperature. Aqueous solutions that are 47.6% HBr by mass form a constant-boiling azeotrope mixture that boils at 124.3 °C (255.7 °F). Boiling less concentrated solutions releases H2O until the constant-boiling mixture composition is reached.

Thionyl chloride is an inorganic compound with the chemical formula SOCl2. It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately 45,000 tonnes per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.
Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting hydrocarbons into fluorocarbons, i.e., it is a fluorination agent. It forms a hydrate and many derivatives.
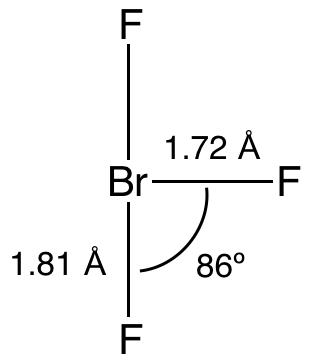
Bromine trifluoride is an interhalogen compound with the formula BrF3. At room temperature, it is a straw-coloured liquid with a pungent odor which decomposes violently on contact with water and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent. It is used to produce uranium hexafluoride (UF6) in the processing and reprocessing of nuclear fuel.
The Beilstein database is the largest database in the field of organic chemistry, in which compounds are uniquely identified by their Beilstein Registry Number. The database covers the scientific literature from 1771 to the present and contains experimentally validated information on millions of chemical reactions and substances from original scientific publications. The electronic database was created from Handbuch der Organischen Chemie, founded by Friedrich Konrad Beilstein in 1881, but has appeared online under a number of different names, including Crossfire Beilstein. Since 2009, the content has been maintained and distributed by Elsevier Information Systems in Frankfurt under the product name "Reaxys".

Terbium(III,IV) oxide, occasionally called tetraterbium heptaoxide, has the formula Tb4O7, though some texts refer to it as TbO1.75. There is some debate as to whether it is a discrete compound, or simply one phase in an interstitial oxide system. Tb4O7 is one of the main commercial terbium compounds, and the only such product containing at least some Tb(IV) (terbium in the +4 oxidation state), along with the more stable Tb(III). It is produced by heating the metal oxalate, and it is used in the preparation of other terbium compounds. Terbium forms three other major oxides: Tb2O3, TbO2, and Tb6O11.

Vanadium(III) chloride describes the inorganic compound with the formula VCl3 and its hydrates. It forms a purple anhydrous form and a green hexahydrate [VCl2(H2O)4]Cl·2H2O. These hygroscopic salts are common precursors to other vanadium(III) complexes and is used as a mild reducing agent.

Nickel nitrate is the inorganic compound Ni(NO3)2 or any hydrate thereof. In the hexahydrate, the nitrate anions are not bonded to nickel. Other hydrates have also been reported: Ni(NO3)2.9H2O, Ni(NO3)2.4H2O, and Ni(NO3)2.2H2O.
Ruthenium hexafluoride, also ruthenium(VI) fluoride (RuF6), is a compound of ruthenium and fluorine and one of the seventeen known binary hexafluorides.

Osmium hexafluoride, also osmium(VI) fluoride, (OsF6) is a compound of osmium and fluorine, and one of the seventeen known binary hexafluorides.

Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound.
Promethium(III) iodide is an inorganic compound, with the chemical formula of PmI3. It is a red radioactive solid with a melting point of 695 °C.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +4, and +3 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. The tetrathioperrhenate anion [ReS4]− is possible.
References
- ↑ Brockhaus ABC Chemie, VEB F. A. Brockhaus Verlag Leipzig 1965, pp. 497–498.
- 1 2 "LibGuides: Chemistry: Gmelin Handbook". University of Texas. 29 July 2022.