
Tamarind is a leguminous tree bearing edible fruit that is indigenous to tropical Africa and naturalized in Asia. The genus Tamarindus is monotypic, meaning that it contains only this species. It belongs to the family Fabaceae.

Teak is a tropical hardwood tree species in the family Lamiaceae. It is a large, deciduous tree that occurs in mixed hardwood forests. Tectona grandis has small, fragrant white flowers arranged in dense clusters (panicles) at the end of the branches. These flowers contain both types of reproductive organs. The large, papery leaves of teak trees are often hairy on the lower surface. Teak wood has a leather-like smell when it is freshly milled and is particularly valued for its durability and water resistance. The wood is used for boat building, exterior construction, veneer, furniture, carving, turnings, and various small projects.

Chamaecyparis obtusa is a species of cypress native to central Japan in East Asia, and widely cultivated in the temperate northern hemisphere for its high-quality timber and ornamental qualities, with many cultivars commercially available.

Phytoalexins are antimicrobial substances, some of which are antioxidative as well. They are defined, not by their having any particular chemical structure or character, but by the fact that they are defensively synthesized de novo by plants that produce the compounds rapidly at sites of pathogen infection. In general phytoalexins are broad spectrum inhibitors; they are chemically diverse, and different chemical classes of compounds are characteristic of particular plant taxa. Phytoalexins tend to fall into several chemical classes, including terpenoids, glycosteroids, and alkaloids; however the term applies to any phytochemicals that are induced by microbial infection.
1-Triacontanol (n-triacontanol) is a fatty alcohol of the general formula C30H62O, also known as melissyl alcohol or myricyl alcohol. It is found in plant cuticle waxes and in beeswax. Triacontanol is a growth stimulant for many plants, most notably roses, in which it rapidly increases the number of basal breaks. 1-Triacontanol is a natural plant growth regulator. It has been widely used to enhance the yield of various crops around the world, mainly in Asia. Triacontanol has been reported to increase the growth of plants by enhancing the rates of photosynthesis, protein biosynthesis, the transport of nutrients in a plant and enzyme activity, reducing complex carbohydrates among many other purposes. The fatty alcohol appears to increase the physiological efficiency of plant cells and boost the potential of the cells responsible for the growth and maturity of a plant.

Cedrus deodara, the deodar cedar, Himalayan cedar, or deodar, is a species of cedar native to the Himalayas.

Umbelliferone, also known as 7-hydroxycoumarin, hydrangine, skimmetine, and beta-umbelliferone, is a natural product of the coumarin family.

Pinosylvin is an organic compound with the formula C6H5CH=CHC6H3(OH)2. A white solid, it is related to trans-stilbene, but with two hydroxy groups on one of the phenyl substituents. It is very soluble in many organic solvents, such as acetone.
Neoflavonoids are a class of polyphenolic compounds. While flavonoids have the 2-phenylchromen-4-one backbone, neoflavonoids have the 4-phenylchromen backbone with no hydroxyl group substitution at position 2.
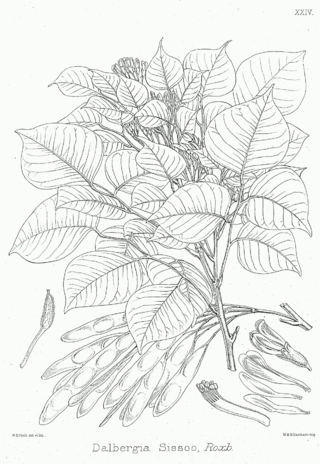
Dalbergia sissoo, known commonly as North Indian rosewood or shisham, is a fast-growing, hardy, deciduous rosewood tree native to the Indian subcontinent and southern Iran. D. sissoo is a large, crooked tree with long, leathery leaves and whitish or pink flowers.

Thujaplicins are a series of tropolone-related chemical substances that have been isolated from the softwoods of the trees of Cupressaceae family. These compounds are known for their antibacterial, antifungal, and antioxidant properties. They were the first natural tropolones to be made synthetically.

Hinokitiol (β-thujaplicin) is a natural monoterpenoid found in the wood of trees in the family Cupressaceae. It is a tropolone derivative and one of the thujaplicins. Hinokitiol is used in oral and skin care products, and is a food additive used in Japan.

Taxodone is a naturally occurring diterpenoid found in Taxodium distichum, Rosmarinus officinalis (rosemary), several salvia species and other plants, along with its oxidized rearrangement product, taxodione. Taxodone and taxodione exhibit anticancer, antibacterial, antioxidant, antifungal, insecticide, and antifeedant activities.
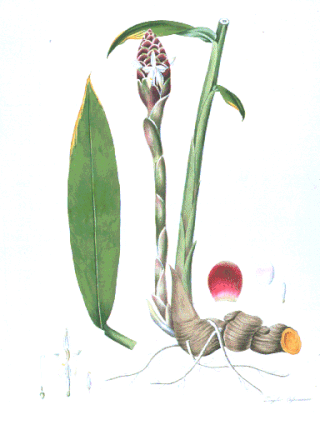
Cassumunar ginger: Zingiber cassumunar, now thought to be a synonym of Zingiber montanum (J.König) Link ex A.Dietr., is a species of plant in the ginger family and is also a relative of galangal. It is called plai (ไพล) in Thailand, in addition to in Isan language and in northern Thai language. The rhizome of variant 'Roxburgh' is used medicinally in massage and even in food in Thailand, and somewhat resembles ginger root or galangal. In aromatherapy, plai oil is used as an essential oil and is believed to ease pain and inflammation. It is also known as ponlei (ពន្លៃ) in Cambodia.

Artocarpus lacucha, also known as monkey jack or monkey fruit, is a tropical evergreen tree species of the family Moraceae. It is distributed throughout the Indian Subcontinent and Southeast Asia. The tree is valued for its wood; its fruit is edible and is believed to have medicinal value. In Northeastern Thailand, the wood is used to make pong lang, a local traditional instrument.
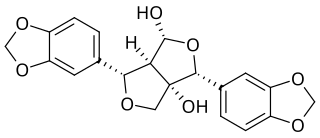
Gummadiol is a lignan hemiacetal. It can be isolated from the heartwood of Gmelina arborea.
Zanthoxylum gilletii, the East African satinwood, is a tree species in the genus Zanthoxylum found in Africa. The fruits are used to produce the spice uzazi.
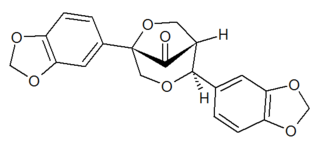
Gmelanone is a lignan found in the heartwood of Gmelina arborea. Arboreol can be transformed by acid catalysis into gmelanone.

Gmelinol is a lignan. (+)-Gmelinol can be isolated from the heartwood of Gmelina arborea. This compound, along with four other chemicals also found in the same species, (+)-7′-O-ethyl arboreol, (+)-paulownin, (+)-epieudesmin and (−)-β-sitosterol, shows antifungal activity against Trametes versicolor.

Trichothecium roseum is a fungus in the division Ascomycota first reported in 1809. It is characterized by its flat and granular colonies which are initially white and develop to be light pink in color. This fungus reproduces asexually through the formation of conidia with no known sexual state. Trichothecium roseum is distinctive from other species of the genus Trichothecium in its characteristic zigzag patterned chained conidia. It is found in various countries worldwide and can grow in a variety of habitats ranging from leaf litter to fruit crops. Trichothecium roseum produces a wide variety of secondary metabolites including mycotoxins, such as roseotoxins and trichothecenes, which can infect and spoil a variety of fruit crops. It can act as both a secondary and opportunistic pathogen by causing pink rot on various fruits and vegetables and thus has an economical impact on the farming industry. Secondary metabolites of T. roseum, specifically Trichothecinol A, are being investigated as potential anti-metastatic drugs. Several agents including harpin, silicon oxide, and sodium silicate are potential inhibitors of T. roseum growth on fruit crops. Trichothecium roseum is mainly a plant pathogen and has yet to show a significant impact on human health.






















