
A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two ions, often of very different sizes, and X is an ion (frequently oxide) that bonds to both ions. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in their oxidation states.

Titanium dioxide, also known as titanium(IV) oxide or titania, is the inorganic compound with the chemical formula TiO
2. When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white, water-insoluble solid, although mineral forms can appear black. As a pigment, it has a wide range of applications, including paint, sunscreen, and food coloring. When used as a food coloring, it has E number E171. World production in 2014 exceeded 9 million tonnes. It has been estimated that titanium dioxide is used in two-thirds of all pigments, and pigments based on the oxide have been valued at $13.2 billion.

Strontium titanate is an oxide of strontium and titanium with the chemical formula SrTiO3. At room temperature, it is a centrosymmetric paraelectric material with a perovskite structure. At low temperatures it approaches a ferroelectric phase transition with a very large dielectric constant ~104 but remains paraelectric down to the lowest temperatures measured as a result of quantum fluctuations, making it a quantum paraelectric. It was long thought to be a wholly artificial material, until 1982 when its natural counterpart—discovered in Siberia and named tausonite—was recognised by the IMA. Tausonite remains an extremely rare mineral in nature, occurring as very tiny crystals. Its most important application has been in its synthesized form wherein it is occasionally encountered as a diamond simulant, in precision optics, in varistors, and in advanced ceramics.

The ABO blood group system is used to denote the presence of one, both, or neither of the A and B antigens on erythrocytes. For human blood transfusions, it is the most important of the 43 different blood type classification systems currently recognized by the International Society of Blood Transfusions (ISBT) as of June 2021. A mismatch in this, or any other serotype, can cause a potentially fatal adverse reaction after a transfusion, or an unwanted immune response to an organ transplant. The associated anti-A and anti-B antibodies are usually IgM antibodies, produced in the first years of life by sensitization to environmental substances such as food, bacteria, and viruses.

Victor Moritz Goldschmidt was a Norwegian mineralogist considered to be the founder of modern geochemistry and crystal chemistry, developer of the Goldschmidt Classification of elements.
Ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the cation and anion gives the distance between the ions in a crystal lattice. Ionic radii are typically given in units of either picometers (pm) or angstroms (Å), with 1 Å = 100 pm. Typical values range from 31 pm (0.3 Å) to over 200 pm (2 Å).

Indium(III) oxide (In2O3) is a chemical compound, an amphoteric oxide of indium.
A definition in semiconductor physics, carrier lifetime is defined as the average time it takes for a minority carrier to recombine. The process through which this is done is typically known as minority carrier recombination.

Lanthanum strontium manganite (LSM or LSMO) is an oxide ceramic material with the general formula La1−xSrxMnO3, where x describes the doping level.
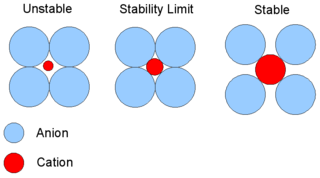
Anions are larger than cations. Large sized anions occupy lattice sites, while small sized cations are found in voids. The ratio of radius of cation to anion is called radius ratio.

Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula CaTiO3). Its name is also applied to the class of compounds which have the same type of crystal structure as CaTiO3 (XIIA2+VIB4+X2−3), known as the perovskite structure. Many different cations can be embedded in this structure, allowing the development of diverse engineered materials.

Calcium titanate is an inorganic compound with the chemical formula CaTiO3. As a mineral, it is called perovskite, named after Russian mineralogist, L. A. Perovski (1792-1856). It is a colourless, diamagnetic solid, although the mineral is often coloured owing to impurities.

Calcium copper titanate (also abbreviated CCTO, for calcium copper titanium oxide) is an inorganic compound with the formula CaCu3Ti4O12. It is noteworthy for its extremely large dielectric constant (effective relative permittivity) of in excess of 10,000 at room temperature.

Geikielite is a magnesium titanium oxide mineral with formula: MgTiO3. It is a member of the ilmenite group. It crystallizes in the trigonal system forming typically opaque, black to reddish black crystals.
Silicate perovskite is either (Mg,Fe)SiO3 or CaSiO3 when arranged in a perovskite structure. Silicate perovskites are not stable at Earth's surface, and mainly exist in the lower part of Earth's mantle, between about 670 and 2,700 km depth. They are thought to form the main mineral phases, together with ferropericlase.

A perovskite solar cell (PSC) is a type of solar cell which includes a perovskite-structured compound, most commonly a hybrid organic-inorganic lead or tin halide-based material, as the light-harvesting active layer. Perovskite materials, such as methylammonium lead halides and all-inorganic cesium lead halide, are cheap to produce and simple to manufacture.
Antiperovskites is a type of crystal structure similar to the perovskite structure that is common in nature. The key difference is that the positions of the cation and anion constituents are reversed in the unit cell structure. In contrast to perovskite, antiperovskite compounds consist of two types of anions coordinated with one type of cation. Antiperovskite compounds are an important class of materials because they exhibit interesting and useful physical properties not found in perovskite materials.
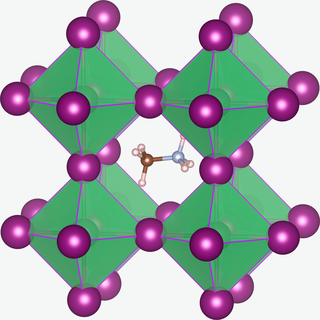
Methylammonium lead halides (MALHs) are solid compounds with perovskite structure and a chemical formula of CH3NH3PbX3, where X = I, Br or Cl. They have potential applications in solar cells, lasers, light-emitting diodes, photodetectors, radiation detectors, scintillator, magneto-optical data storage and hydrogen production.

Perovskite nanocrystals are a class of semiconductor nanocrystals, which exhibit unique characteristics that separate them from traditional quantum dots. Perovskite nanocrystals have an ABX3 composition where A = cesium, methylammonium (MA), or formamidinium (FA); B = lead or tin; and X = chloride, bromide, or iodide.

Isaac B. Bersuker is a Soviet-Moldоvan-American theoretical physicist and quantum chemist whose principal research is in chemical physics, solid-state physics, and theoretical chemistry. Known for his "life-long years of experience in theoretical chemistry" working on the electronic structure and properties of coordination compounds, Isaac B. Bersuker is “one of the most widely recognized authorities” in the theory of the Jahn-Teller effect (JTE) and the pseudo-Jahn-Teller effect (PJTE). His accomplishments include explaining the polarization of the atomic core in Rydberg atoms, the effect of tunneling splitting in molecules and solids with a strong JTE, and the discovery of the PJTE origin of ferroelectricity in cubic perovskites. Known as the leading expert in JTE and PJTE, Bersuker is the permanent Chairman of the International Steering Committee of the Jahn-Teller symposia. His present affiliation is with the Oden Institute for Computational Engineering and Science of the Department of Chemistry of the University of Texas at Austin.




















