
The entorhinal cortex (EC) is an area of the brain's allocortex, located in the medial temporal lobe, whose functions include being a widespread network hub for memory, navigation, and the perception of time. The EC is the main interface between the hippocampus and neocortex. The EC-hippocampus system plays an important role in declarative (autobiographical/episodic/semantic) memories and in particular spatial memories including memory formation, memory consolidation, and memory optimization in sleep. The EC is also responsible for the pre-processing (familiarity) of the input signals in the reflex nictitating membrane response of classical trace conditioning; the association of impulses from the eye and the ear occurs in the entorhinal cortex.
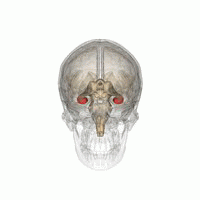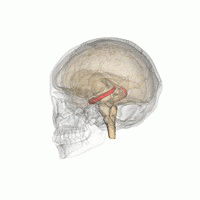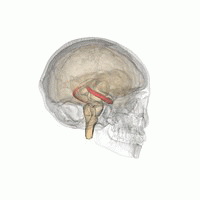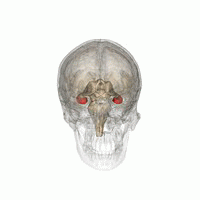
The hippocampus is a major component of the brain of humans and other vertebrates. Humans and other mammals have two hippocampi, one in each side of the brain. The hippocampus is part of the limbic system, and plays important roles in the consolidation of information from short-term memory to long-term memory, and in spatial memory that enables navigation. The hippocampus is located in the allocortex, with neural projections into the neocortex, in humans as well as other primates. The hippocampus, as the medial pallium, is a structure found in all vertebrates. In humans, it contains two main interlocking parts: the hippocampus proper, and the dentate gyrus.
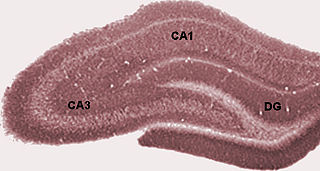
The dentate gyrus (DG) is part of the hippocampal formation in the temporal lobe of the brain, which also includes the hippocampus and the subiculum. The dentate gyrus is part of the hippocampal trisynaptic circuit and is thought to contribute to the formation of new episodic memories, the spontaneous exploration of novel environments and other functions.

In cognitive psychology and neuroscience, spatial memory is a form of memory responsible for the recording and recovery of information needed to plan a course to a location and to recall the location of an object or the occurrence of an event. Spatial memory is necessary for orientation in space. Spatial memory can also be divided into egocentric and allocentric spatial memory. A person's spatial memory is required to navigate around a familiar city. A rat's spatial memory is needed to learn the location of food at the end of a maze. In both humans and animals, spatial memories are summarized as a cognitive map.

A place cell is a kind of pyramidal neuron in the hippocampus that becomes active when an animal enters a particular place in its environment, which is known as the place field. Place cells are thought to act collectively as a cognitive representation of a specific location in space, known as a cognitive map. Place cells work with other types of neurons in the hippocampus and surrounding regions to perform this kind of spatial processing. They have been found in a variety of animals, including rodents, bats, monkeys and humans.

A cognitive map is a type of mental representation which serves an individual to acquire, code, store, recall, and decode information about the relative locations and attributes of phenomena in their everyday or metaphorical spatial environment. The concept was introduced by Edward Tolman in 1948. He tried to explain the behavior of rats that appeared to learn the spatial layout of a maze, and subsequently the concept was applied to other animals, including humans. The term was later generalized by some researchers, especially in the field of operations research, to refer to a kind of semantic network representing an individual's personal knowledge or schemas.

Motion perception is the process of inferring the speed and direction of elements in a scene based on visual, vestibular and proprioceptive inputs. Although this process appears straightforward to most observers, it has proven to be a difficult problem from a computational perspective, and difficult to explain in terms of neural processing.
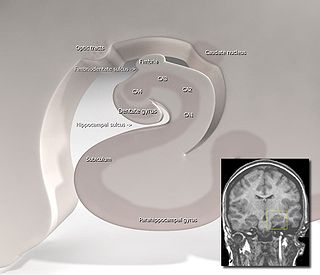
The subiculum is the most inferior component of the hippocampal formation. It lies between the entorhinal cortex and the CA1 subfield of the hippocampus proper.
Head direction (HD) cells are neurons found in a number of brain regions that increase their firing rates above baseline levels only when the animal's head points in a specific direction. They have been reported in rats, monkeys, mice, chinchillas and bats, but are thought to be common to all mammals, perhaps all vertebrates and perhaps even some invertebrates, and to underlie the "sense of direction". When the animal's head is facing in the cell's "preferred firing direction" these neurons fire at a steady rate, but firing decreases back to baseline rates as the animal's head turns away from the preferred direction.

Path integration is the method thought to be used by animals for dead reckoning.

The hippocampal formation is a compound structure in the medial temporal lobe of the brain. It forms a c-shaped bulge on the floor of the temporal horn of the lateral ventricle. There is no consensus concerning which brain regions are encompassed by the term, with some authors defining it as the dentate gyrus, the hippocampus proper and the subiculum; and others including also the presubiculum, parasubiculum, and entorhinal cortex. The hippocampal formation is thought to play a role in memory, spatial navigation and control of attention. The neural layout and pathways within the hippocampal formation are very similar in all mammals.

In the brain, the perforant path or perforant pathway provides a connectional route from the entorhinal cortex to all fields of the hippocampal formation, including the dentate gyrus, all CA fields, and the subiculum.

Michael Hasselmo is an American neuroscientist and professor in the Department of Psychological and Brain Sciences at Boston University. He is the director of the Center for Systems Neuroscience and is editor-in-chief of Hippocampus (journal). Hasselmo studies oscillatory dynamics and neuromodulatory regulation in cortical mechanisms for memory guided behavior and spatial navigation using a combination of neurophysiological and behavioral experiments in conjunction with computational modeling. In addition to his peer-reviewed publications, Hasselmo wrote the book How We Remember: Brain Mechanisms of Episodic Memory.

Hippocampus anatomy describes the physical aspects and properties of the hippocampus, a neural structure in the medial temporal lobe of the brain. It has a distinctive, curved shape that has been likened to the sea-horse monster of Greek mythology and the ram's horns of Amun in Egyptian mythology. This general layout holds across the full range of mammalian species, from hedgehog to human, although the details vary. For example, in the rat, the two hippocampi look similar to a pair of bananas, joined at the stems. In primate brains, including humans, the portion of the hippocampus near the base of the temporal lobe is much broader than the part at the top. Due to the three-dimensional curvature of this structure, two-dimensional sections such as shown are commonly seen. Neuroimaging pictures can show a number of different shapes, depending on the angle and location of the cut.

Boundary cells are neurons found in the hippocampal formation that respond to the presence of an environmental boundary at a particular distance and direction from an animal. The existence of cells with these firing characteristics were first predicted on the basis of properties of place cells. Boundary cells were subsequently discovered in several regions of the hippocampal formation: the subiculum, presubiculum and entorhinal cortex.

Edvard Ingjald Moser is a Norwegian psychologist and neuroscientist, who is a professor at the Norwegian University of Science and Technology (NTNU) in Trondheim. In 2005, he and his then-wife May-Britt Moser discovered grid cells in the brain's medial entorhinal cortex. Grid cells are specialized neurons that provide the brain with a coordinate system and a metric for space. In 2018, he discovered a neural network that expresses a person's sense of time in experiences and memories located in the brain's lateral entorhinal cortex.

May-Britt Moser is a Norwegian psychologist and neuroscientist, who is a Professor of Psychology and Neuroscience at the Norwegian University of Science and Technology (NTNU). She and her former husband, Edvard Moser, shared half of the 2014 Nobel Prize in Physiology or Medicine, awarded for work concerning the grid cells in the entorhinal cortex, as well as several additional space-representing cell types in the same circuit that make up the positioning system in the brain. Together with Edvard Moser she established the Moser research environment at NTNU, which they lead. Since 2012 she has headed the Centre for Neural Computation.

John O'Keefe, is an American-British neuroscientist, psychologist and a professor at the Sainsbury Wellcome Centre for Neural Circuits and Behaviour and the Research Department of Cell and Developmental Biology at University College London. He discovered place cells in the hippocampus, and that they show a specific kind of temporal coding in the form of theta phase precession. He shared the Nobel Prize in Physiology or Medicine in 2014, together with May-Britt Moser and Edvard Moser; he has received several other awards. He has worked at University College London for his entire career, but also held a part-time chair at the Norwegian University of Science and Technology at the behest of his Norwegian collaborators, the Mosers.

Phase precession is a neurophysiological process in which the time of firing of action potentials by individual neurons occurs progressively earlier in relation to the phase of the local field potential oscillation with each successive cycle. In place cells, a type of neuron found in the hippocampal region of the brain, phase precession is believed to play a major role in the neural coding of information. John O'Keefe, who later shared the 2014 Nobel Prize in Physiology or Medicine for his discovery that place cells help form a "map" of the body's position in space, co-discovered phase precession with Michael Recce in 1993.
Lisa Giocomo is an American neuroscientist who is a Professor in the Department of Neurobiology at Stanford University School of Medicine. Giocomo probes the molecular and cellular mechanisms underlying cortical neural circuits involved in spatial navigation and memory.



















