Related Research Articles

In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6H5, and is often represented by the symbol Ph. The phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. A phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, the phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring.
In organic chemistry, an aryl halide is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications.
In electrophilic aromatic substitution reactions, existing substituent groups on the aromatic ring influence the overall reaction rate or have a directing effect on positional isomer of the products that are formed.

Bromobenzene is an aryl bromide and the simplest of the bromobenzenes, consisting of a benzene ring substituted with one bromine atom. Its chemical formula is C6H5Br. It is a colourless liquid although older samples can appear yellow. It is a reagent in organic synthesis.

Chlorobenzene (abbreviated PhCl) is an aryl chloride and the simplest of the chlorobenzenes, consisting of a benzene ring substituted with one chlorine atom. Its chemical formula is C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide.

The Masamune-Bergman cyclization or Masamune-Bergman reaction or Masamune-Bergman cycloaromatization is an organic reaction and more specifically a rearrangement reaction taking place when an enediyne is heated in presence of a suitable hydrogen donor. It is the most famous and well-studied member of the general class of cycloaromatization reactions. It is named for Japanese-American chemist Satoru Masamune and American chemist Robert G. Bergman. The reaction product is a derivative of benzene.

Iodobenzene is an aryl iodide and the simplest of the iodobenzenes, consisting of a benzene ring substituted with one iodine atom. Its chemical formula is C6H5I. It is useful as a synthetic intermediate in organic chemistry. It is a volatile colorless liquid, although aged samples appear yellowish.
Bromobenzenes are a group of aryl bromides/halobenzenes consisting of one or more bromine atoms as substituents on a benzene core. They have the formula C6H6–nBrn, where n = 1–6 is the number of bromine atoms. Depending on the number of bromine substituents, there may be several constitutional isomers possible.
Chlorobenzenes are a group of aryl chlorides/halobenzenes consisting of one or more chlorine atoms as substituents on a benzene core. They have the formula C6H6–nCln, where n = 1–6 is the number of chlorine atoms. Depending on the number of chlorine substituents, there may be several constitutional isomers possible.
Iodobenzenes are a group of aryl iodides/halobenzenes consisting of one or more iodine atoms as substituents on a benzene core. They have the formula C6H6–nIn, where n = 1–6 is the number of iodine atoms. Depending on the number of bromine substituents, there may be several constitutional isomers possible.
Fluorobenzene is an aryl fluoride and the simplest of the fluorobenzenes, with the formula C6H5F, often abbreviated PhF. A colorless liquid, it is a precursor to many fluorophenyl compounds.

NanoPutians are a series of organic molecules whose structural formulae resemble human forms. James Tour's research group designed and synthesized these compounds in 2003 as a part of a sequence on chemical education for young students. The compounds consist of two benzene rings connected via a few carbon atoms as the body, four acetylene units each carrying an alkyl group at their ends which represents the hands and legs, and a 1,3-dioxolane ring as the head. Tour and his team at Rice University used the NanoPutians in their NanoKids educational outreach program. The goal of this program was to educate children in the sciences in an effective and enjoyable manner. They have made several videos featuring the NanoPutians as anthropomorphic animated characters.
Organoiodine chemistry is the study of the synthesis and properties of organoiodine compounds, or organoiodides, organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine hormones are organoiodine compounds that are required for health and the reason for government-mandated iodization of salt.
Unlike its lighter congeners, the halogen iodine forms a number of stable organic compounds, in which iodine exhibits higher formal oxidation states than -1 or coordination number exceeding 1. These are the hypervalent organoiodines, often called iodanes after the IUPAC rule used to name them.
Bromochlorobenzenes are mixed aryl halides consisting bromine and chlorine as substituents on a benzene ring.

Pentaphenylantimony is an organoantimony compound containing five phenyl groups attached to one antimony atom. It has formula Sb(C6H5)5 (or SbPh5).
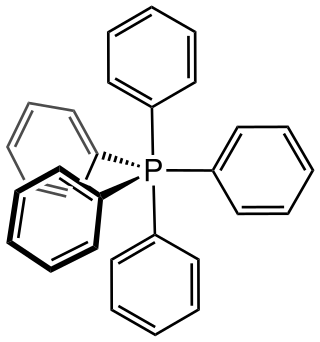
Pentaphenylphosphorus is an organic phosphorane containing five phenyl groups connected to a central phosphorus atom. The phosphorus atom is considered to be in the +5 oxidation state. The chemical formula could be written as P(C6H5)5 or Ph5P, where Ph represents the phenyl group. It was discovered and reported in 1949 by Georg Wittig.
An arsinide, arsanide, dihydridoarsenate(1−) or arsanyl compound is a chemical derivative of arsine, where one hydrogen atom is replaced with a metal or cation. The arsinide ion has formula AsH−2. It can be considered as a ligand with name arsenido or arsanido. Researchers are unenthusiastic about studying arsanyl compounds, because of the toxic chemicals, and their instability. The IUPAC names are arsanide and dihydridoarsenate(1−). For the ligand the name is arsanido. The neutral −AsH2 group is termed arsanyl.
Fluorobenzenes are a group of aryl fluorides/halobenzenes consisting of one or more fluorine atoms as substituents on a benzene core. They have the formula C6H6–nFn, where n = 1–6 is the number of fluorine atoms. Depending on the number of fluorine substituents, there may be several constitutional isomers possible.
References
- ↑ Adrio, Luis A.; Miguez, Jose M. Antelo; Hii, K. K. (M.) (2009). "Synthesis of Terphenyls". Organic Preparations and Procedures International . 41 (5): 331–358. doi:10.1080/00304940903163632.