Related Research Articles

Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.

Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride.

Lonsdaleite, also called hexagonal diamond in reference to the crystal structure, is an allotrope of carbon with a hexagonal lattice, as opposed to the cubical lattice of conventional diamond. It is found in nature in meteorite debris; when meteors containing graphite strike the Earth, the immense heat and stress of the impact transforms the graphite into diamond, but retains graphite's hexagonal crystal lattice. Lonsdaleite was first identified in 1967 from the Canyon Diablo meteorite, where it occurs as microscopic crystals associated with ordinary diamond.

β-Carbon nitride (beta-carbon nitride), β-C3N4, is a superhard material predicted to be harder than diamond.
Borazon is a brand name of a cubic form of boron nitride (cBN). Its color ranges from black to brown and gold, depending on the chemical bond. It is one of the hardest known materials, along with various forms of diamond and other kinds of boron nitride. Borazon is a crystal created by heating equal quantities of boron and nitrogen at temperatures greater than 1800 °C (3300 °F) at 7 GPa.

Boron carbide (chemical formula approximately B4C) is an extremely hard boron–carbon ceramic, a covalent material used in tank armor, bulletproof vests, engine sabotage powders, as well as numerous industrial applications. With a Vickers hardness of >30 GPa, it is one of the hardest known materials, behind cubic boron nitride and diamond.
A superhard material is a material with a hardness value exceeding 40 gigapascals (GPa) when measured by the Vickers hardness test. They are virtually incompressible solids with high electron density and high bond covalency. As a result of their unique properties, these materials are of great interest in many industrial areas including, but not limited to, abrasives, polishing and cutting tools, disc brakes, and wear-resistant and protective coatings.

Diamond is the allotrope of carbon in which the carbon atoms are arranged in the specific type of cubic lattice called diamond cubic. It is a crystal that is transparent to opaque and which is generally isotropic. Diamond is the hardest naturally occurring material known. Yet, due to important structural brittleness, bulk diamond's toughness is only fair to good. The precise tensile strength of bulk diamond is little known; however, compressive strength up to 60 GPa has been observed, and it could be as high as 90–100 GPa in the form of micro/nanometer-sized wires or needles, with a corresponding maximum tensile elastic strain in excess of 9%. The anisotropy of diamond hardness is carefully considered during diamond cutting. Diamond has a high refractive index (2.417) and moderate dispersion (0.044) properties that give cut diamonds their brilliance. Scientists classify diamonds into four main types according to the nature of crystallographic defects present. Trace impurities substitutionally replacing carbon atoms in a diamond's crystal structure, and in some cases structural defects, are responsible for the wide range of colors seen in diamond. Most diamonds are electrical insulators and extremely efficient thermal conductors. Unlike many other minerals, the specific gravity of diamond crystals (3.52) has rather small variation from diamond to diamond.
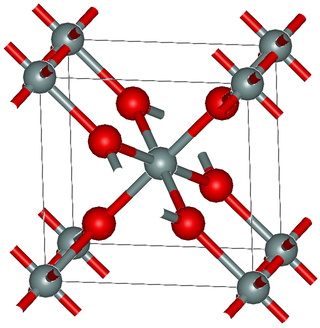
Stishovite is an extremely hard, dense tetragonal form (polymorph) of silicon dioxide. It is very rare on the Earth's surface; however, it may be a predominant form of silicon dioxide in the Earth, especially in the lower mantle.

Aggregated diamond nanorods, or ADNRs, are a nanocrystalline form of diamond, also known as nanodiamond or hyperdiamond.

Silicon nitride is a chemical compound of the elements silicon and nitrogen. Si
3N
4 is the most thermodynamically stable and commercially important of the silicon nitrides, and the term ″Silicon nitride″ commonly refers to this specific composition. It is a white, high-melting-point solid that is relatively chemically inert, being attacked by dilute HF and hot H
3PO
4. It is very hard. It has a high thermal stability with strong optical nonlinearities for all-optical applications.

Magnesium nitride, which possesses the chemical formula Mg3N2, is an inorganic compound of magnesium and nitrogen. At room temperature and pressure it is a greenish yellow powder.

Rhenium diboride (ReB2) is a synthetic high-hardness material that was first synthesized in 1962. The compound is formed from a mixture of rhenium, noted for its resistance to high pressure, and boron, which forms short, strong covalent bonds with rhenium. It has regained popularity in recent times in hopes of finding a material that possesses hardness comparable to that of diamond.

Covalent superconductors are superconducting materials where the atoms are linked by covalent bonds. The first such material was boron-doped synthetic diamond grown by the high-pressure high-temperature (HPHT) method. The discovery had no practical importance, but surprised most scientists as superconductivity had not been observed in covalent semiconductors, including diamond and silicon.
Aluminium magnesium boride or Al3Mg3B56, colloquially known as BAM, is a chemical compound of aluminium, magnesium and boron. Whereas its nominal formula is AlMgB14, the chemical composition is closer to Al0.75Mg0.75B14. It is a ceramic alloy that is highly resistive to wear and has an extremely low coefficient of sliding friction, reaching a record value of 0.04 in unlubricated and 0.02 in lubricated AlMgB14−TiB2 composites. First reported in 1970, BAM has an orthorhombic structure with four icosahedral B12 units per unit cell. This ultrahard material has a coefficient of thermal expansion comparable to that of other widely used materials such as steel and concrete.

Ruthenium borides are compounds of ruthenium and boron. Their most remarkable property is potentially high hardness. Vickers hardness HV = 50 GPa was reported for thin films composed of RuB2 and Ru2B3 phases. This value is significantly higher than those of bulk RuB2 or Ru2B3, but it has to be confirmed independently, as measurements on superhard materials are intrinsically difficult. For example, note that the initial report on extreme hardness of related material rhenium diboride was probably too optimistic.

Boron can be prepared in several crystalline and amorphous forms. Well known crystalline forms are α-rhombohedral (α-R), β-rhombohedral (β-R), and β-tetragonal (β-T). In special circumstances, boron can also be synthesized in the form of its α-tetragonal (α-T) and γ-orthorhombic (γ) allotropes. Two amorphous forms, one a finely divided powder and the other a glassy solid, are also known. Although at least 14 more allotropes have been reported, these other forms are based on tenuous evidence or have not been experimentally confirmed, or are thought to represent mixed allotropes, or boron frameworks stabilized by impurities. Whereas the β-rhombohedral phase is the most stable and the others are metastable, the transformation rate is negligible at room temperature, and thus all five phases can exist at ambient conditions. Amorphous powder boron and polycrystalline β-rhombohedral boron are the most common forms. The latter allotrope is a very hard grey material, about ten percent lighter than aluminium and with a melting point (2080 °C) several hundred degrees higher than that of steel.

Boron nitride nanotubes (BNNTs) are a polymorph of boron nitride. They were predicted in 1994 and experimentally discovered in 1995. Structurally they are similar to carbon nanotubes, which are cylinders with sub-micrometer diameters and micrometer lengths, except that carbon atoms are alternately substituted by nitrogen and boron atoms. However, the properties of BN nanotubes are very different: whereas carbon nanotubes can be metallic or semiconducting depending on the rolling direction and radius, a BN nanotube is an electrical insulator with a bandgap of ~5.5 eV, basically independent of tube chirality and morphology. In addition, a layered BN structure is much more thermally and chemically stable than a graphitic carbon structure. BNNTs have unique physical and chemical properties, when compared to Carbon Nanotubes (CNTs) providing a very wide range of commercial and scientific applications. Although BNNTs and CNTs share similar tensile strength properties of circa 100 times stronger than steel and 50 times stronger than industrial-grade carbon fibre, BNNTs can withstand high temperatures of up to 900 °C. as opposed to CNTs which remain stable up to temperatures of 400 °C, and are also capable of absorbing radiation. BNNTS are packed with physicochemical features including high hydrophobicity and considerable hydrogen storage capacity and they are being investigated for possible medical and biomedical applications, including gene delivery, drug delivery, neutron capture therapy, and more generally as biomaterials BNNTs are also superior to CNTs in the way they bond to polymers giving rise to many new applications and composite materials.
Cutting tool materials are materials that are used to make cutting tools which are used in machining but not other cutting tools like knives or punches.
Francis Pettit Bundy was an American physicist, known as a member of General Electric's team of researchers that in December 1954 created diamond chips by applying ultra high pressure to graphite with iron sulfide as a catalyst.
References
- ↑ Komatsu, T.; Samedima, M.; Awano, T.; Kakadate, Y.; Fujiwara, S. (1999). "Creation of Superhard B–C–N Heterodiamond Using an Advanced Shock Wave Compression Technology". Journal of Materials Processing Technology. 85 (1–3): 69–73. doi:10.1016/S0924-0136(98)00263-5.
- ↑ Solozhenko, V. L.; Andrault, D.; Fiquet, G.; Mezouar, M.; Rubie, D. C. (2001). "Synthesis of Superhard Cubic BC2N". Applied Physics Letters. 78 (10): 1385–1387. Bibcode:2001ApPhL..78.1385S. doi:10.1063/1.1337623.