
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity".
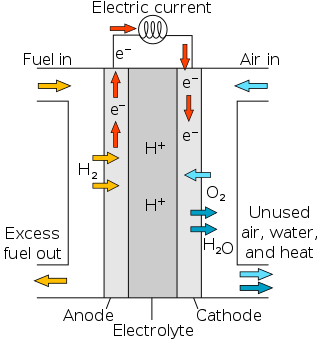
Proton-exchange membrane fuel cells (PEMFC), also known as polymer electrolyte membrane (PEM) fuel cells, are a type of fuel cell being developed mainly for transport applications, as well as for stationary fuel-cell applications and portable fuel-cell applications. Their distinguishing features include lower temperature/pressure ranges and a special proton-conducting polymer electrolyte membrane. PEMFCs generate electricity and operate on the opposite principle to PEM electrolysis, which consumes electricity. They are a leading candidate to replace the aging alkaline fuel-cell technology, which was used in the Space Shuttle.
A "photoelectrochemical cell" is one of two distinct classes of device. The first produces electrical energy similarly to a dye-sensitized photovoltaic cell, which meets the standard definition of a photovoltaic cell. The second is a photoelectrolytic cell, that is, a device which uses light incident on a photosensitizer, semiconductor, or aqueous metal immersed in an electrolytic solution to directly cause a chemical reaction, for example to produce hydrogen via the electrolysis of water.

The Sabatier reaction or Sabatier process produces methane and water from a reaction of hydrogen with carbon dioxide at elevated temperatures and pressures in the presence of a nickel catalyst. It was discovered by the French chemists Paul Sabatier and Jean-Baptiste Senderens in 1897. Optionally, ruthenium on alumina makes a more efficient catalyst. It is described by the following exothermic reaction:
A proton-exchange membrane, or polymer-electrolyte membrane (PEM), is a semipermeable membrane generally made from ionomers and designed to conduct protons while acting as an electronic insulator and reactant barrier, e.g. to oxygen and hydrogen gas. This is their essential function when incorporated into a membrane electrode assembly (MEA) of a proton-exchange membrane fuel cell or of a proton-exchange membrane electrolyser: separation of reactants and transport of protons while blocking a direct electronic pathway through the membrane.
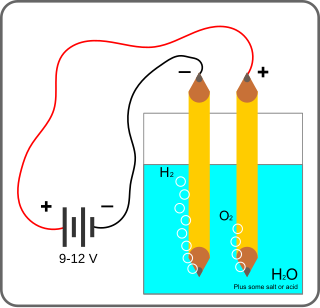
Electrolysis of water is using electricity to split water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, but must be kept apart from the oxygen as the mixture would be extremely explosive. Separately pressurised into convenient 'tanks' or 'gas bottles', hydrogen can be used for oxyhydrogen welding and other applications, as the hydrogen / oxygen flame can reach approximately 2,800°C.
Oxygenevolution is the process of generating molecular oxygen (O2) by a chemical reaction, usually from water. Oxygen evolution from water is effected by oxygenic photosynthesis, electrolysis of water, and thermal decomposition of various oxides. The biological process supports aerobic life. When relatively pure oxygen is required industrially, it is isolated by distilling liquefied air.
Gas diffusion electrodes (GDE) are electrodes with a conjunction of a solid, liquid and gaseous interface, and an electrical conducting catalyst supporting an electrochemical reaction between the liquid and the gaseous phase.

An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst can be heterogeneous such as a platinized electrode. Homogeneous electrocatalysts, which are soluble, assist in transferring electrons between the electrode and reactants, and/or facilitate an intermediate chemical transformation described by an overall half reaction. Major challenges in electrocatalysts focus on fuel cells.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
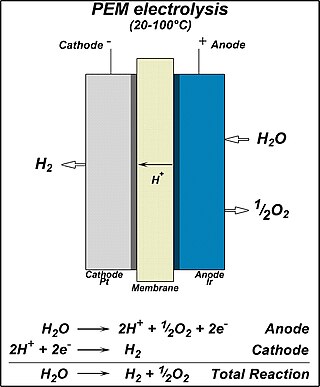
Proton exchange membrane(PEM) electrolysis is the electrolysis of water in a cell equipped with a solid polymer electrolyte (SPE) that is responsible for the conduction of protons, separation of product gases, and electrical insulation of the electrodes. The PEM electrolyzer was introduced to overcome the issues of partial load, low current density, and low pressure operation currently plaguing the alkaline electrolyzer. It involves a proton-exchange membrane.
Alkaline water electrolysis is a type of electrolyzer that is characterized by having two electrodes operating in a liquid alkaline electrolyte. Commonly, a solution of potassium hydroxide (KOH) or sodium hydroxide (NaOH) at 25-40 wt% is used. These electrodes are separated by a diaphragm, separating the product gases and transporting the hydroxide ions (OH−) from one electrode to the other. A recent comparison showed that state-of-the-art nickel based water electrolyzers with alkaline electrolytes lead to competitive or even better efficiencies than acidic polymer electrolyte membrane water electrolysis with platinum group metal based electrocatalysts.

Mixed conductors, also known as mixed ion-electron conductors(MIEC), are a single-phase material that has significant conduction ionically and electronically. Due to the mixed conduction, a formally neutral species can transport in a solid and therefore mass storage and redistribution are enabled. Mixed conductors are well known in conjugation with high-temperature superconductivity and are able to capacitate rapid solid-state reactions.
Yang Shao-Horn is a Chinese American scholar, Professor of Mechanical Engineering and Materials Science and Engineering and a member of Research Laboratory of Electronics at the Massachusetts Institute of Technology. She is known for research on understanding and controlling of processes for storing electrons in chemical bonds towards zero-carbon energy and chemicals.

Water oxidation catalysis (WOC) is the acceleration (catalysis) of the conversion of water into oxygen and protons:
The Virtual breakdown mechanism is a concept in the field of electrochemistry. In electrochemical reactions, when the cathode and the anode are close enough to each other, the double layer of the regions from the two electrodes is overlapped, forming a large electric field uniformly distributed inside the entire electrode gap. Such high electric fields can significantly enhance the ion migration inside bulk solutions and thus increase the entire reaction rate, akin to the "breakdown" of the reactant(s). However, it is fundamentally different from the traditional "breakdown".
Electro-oxidation(EO or EOx), also known as anodic oxidation or electrochemical oxidation (EC), is a technique used for wastewater treatment, mainly for industrial effluents, and is a type of advanced oxidation process (AOP). The most general layout comprises two electrodes, operating as anode and cathode, connected to a power source. When an energy input and sufficient supporting electrolyte are provided to the system, strong oxidizing species are formed, which interact with the contaminants and degrade them. The refractory compounds are thus converted into reaction intermediates and, ultimately, into water and CO2 by complete mineralization.
In chemistry, the oxygen reduction reaction refers to the reduction half reaction whereby O2 is reduced to water or hydrogen peroxide. In fuel cells, the reduction to water is preferred because the current is higher. The oxygen reduction reaction is well demonstrated and highly efficient in nature.

Anion exchange membrane(AEM) electrolysis is the electrolysis of water that utilises a semipermeable membrane that conducts hydroxide ions (OH−) called an anion exchange membrane. Like a proton-exchange membrane (PEM), the membrane separates the products, provides electrical insulation between electrodes, and conducts ions. Unlike PEM, AEM conducts hydroxide ions. The major advantage of AEM water electrolysis is that a high-cost noble metal catalyst is not required, low-cost transition metal catalyst can be used instead. AEM electrolysis is similar to alkaline water electrolysis, which uses a non-ion-selective separator instead of an anion-exchange membrane.











