Related Research Articles

Hornblende is a complex inosilicate series of minerals. It is not a recognized mineral in its own right, but the name is used as a general or field term, to refer to a dark amphibole. Hornblende minerals are common in igneous and metamorphic rocks.

Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, and another occurs naturally as the mineral gypsum. It has many uses in industry. All forms are white solids that are poorly soluble in water. Calcium sulfate causes permanent hardness in water.

Afwillite is a calcium hydroxide nesosilicate mineral with formula Ca3(SiO3OH)2·2H2O. It occurs as glassy, colorless to white prismatic monoclinic crystals. Its Mohs scale hardness is between 3 and 4. It occurs as an alteration mineral in contact metamorphism of limestone. It occurs in association with apophyllite, natrolite, thaumasite, merwinite, spurrite, gehlenite, ettringite, portlandite, hillebrandite, foshagite, brucite and calcite.
Dicalcium phosphate is the calcium phosphate with the formula CaHPO4 and its dihydrate. The "di" prefix in the common name arises because the formation of the HPO42– anion involves the removal of two protons from phosphoric acid, H3PO4. It is also known as dibasic calcium phosphate or calcium monohydrogen phosphate. Dicalcium phosphate is used as a food additive, it is found in some toothpastes as a polishing agent and is a biomaterial.

Xonotlite, or eakleite, is a mineral of general formula Ca6Si6O17(OH)2 named by the German mineralogist Karl Friedrich August Rammelsberg in 1866. The name originates from its discovery locality, Tetela de Xonotla, Puebla, Mexico. Although it was discovered in 1866, it was first described in 1959. It is approved by the IMA, but it is a grandfathered species, meaning the name supposedly represents a valid species til this day.

Ettringite is a hydrous calcium aluminium sulfate mineral with formula: Ca6Al2(SO4)3(OH)12·26H2O. It is a colorless to yellow mineral crystallizing in the trigonal system. The prismatic crystals are typically colorless, turning white on partial dehydration. It is part of the ettringite-group which includes other sulfates such as thaumasite and bentorite.

Ikaite is the mineral name for the hexahydrate of calcium carbonate, CaCO3·6H2O. Ikaite tends to form very steep or spiky pyramidal crystals, often radially arranged, of varied sizes from thumbnail size aggregates to gigantic salient spurs. It is only found in a metastable state and decomposes rapidly by losing most of its water content once removed from near-freezing water. This "melting mineral" is more commonly known through its pseudomorphs.
Partheite or parthéite is a calcium aluminium silicate and a member of the zeolite group of minerals, a group of silicates with large open channels throughout the crystal structure, which allow passage of liquids and gasses through the mineral. It was first discovered in 1979 in rodingitic dikes in an ophiolite zone of the Taurus Mountains in southwest Turkey. The second discovery occurred in gabbro-pegmatites in the Ural Mountains, Russia. Since its discovery and naming, the chemical formula for partheite has been revised from CaAl2Si2O8•2H2O to include not only water but hydroxyl groups as well. The framework of the mineral is interrupted due to these hydroxyl groups attaching themselves to aluminum centered oxygen tetrahedra. This type of interrupted framework is known in only one other zeolite, the mineral roggianite. As a silicate based mineral with the properties of a zeolite, partheite was first described as zeolite-like in 1984 and listed as a zeolite in 1985. Partheite and lawsonite are polymorphs. Associated minerals include prehnite, thomsonite, augite, chlorite and tremolite.

Sodium metaborate is a chemical compound of sodium, boron, and oxygen with formula NaBO
2. However, the metaborate ion is trimeric in the anhydrous solid, therefore a more correct formula is Na3B3O6 or (Na+)3[B3O6]3−. The formula can be written also as Na
2O·B
2O
3 to highlight the relation to the main oxides of sodium and boron. The name is also applied to several hydrates whose formulas can be written NaBO2·nH2O for various values of n.

Jennite is a calcium silicate hydrate mineral of general chemical formula: Ca9Si6O18(OH)6·8H2O.
Cesanite is the end member of the apatite-wilkeite-ellestadite series that substitutes all of apatite's phosphate ions with sulfate ions and balances the difference in charge by replacing several calcium ions with sodium ions. Currently very few sites bearing cesanite have been found and are limited to a geothermal field in Cesano, Italy from which its name is derived, Măgurici Cave in Romania, and in the San Salvador Island caves in the Bahamas.

Picropharmacolite, Ca4Mg(AsO3OH)2(AsO4)2·11H2O, is a rare arsenate mineral. It was named in 1819 from the Greek for bitter, in allusion to its magnesium content, and its chemical similarity to pharmacolite. The mineral irhtemite, Ca4Mg(AsO3OH)2(AsO4)2·4H2O, has the same composition as picropharmacolite, except that it has only four water molecules per formula unit, instead of eleven. It may be formed by the dehydration of picropharmacolite.
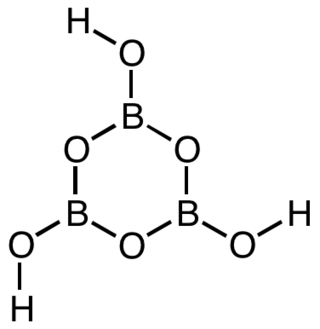
Metaboric acid is the name for a family of inorganic compounds with the same empirical formula HBO2. that differ in their molecular structure. They are colourless water-soluble solids formed by the dehydration or decomposition of boric acid.

Ruizite is a sorosilicate mineral with formula Ca2Mn2Si4O11(OH)4·2H2O. It was discovered at the Christmas mine in Christmas, Arizona, and described in 1977. The mineral is named for discoverer Joe Ana Ruiz.

Junitoite is a mineral with formula CaZn2Si2O7·H2O. It was discovered at the Christmas mine in Christmas, Arizona, and described in 1976. The mineral is named for mineral chemist Jun Ito (1926–1978).

A metaborate is an anion (negative ion) consisting of boron and oxygen, with empirical formula BO−2; or any salt with such anions, such as sodium metaborate, Na+[BO2]− or calcium metaborate Ca2+([BO2]−)2. It is one of the boron oxoanions or borates

Lindgrenite is an uncommon copper molybdate mineral with formula: Cu3(MoO4)2(OH)2. It occurs as tabular to platey monoclinic green to yellow green crystals.

Bicchulite has an ideal chemical formula of 2CaO•Al2O2•SiO2•H2O, which was formularized from the hydrothermal synthesis of synthetic gehlenite. Also, bicchulite was sighted in the mines of Japan with related minerals. This sodalite-type structured bicchulite has an uncommon ratio of aluminium to silicon, causing difficulties deciphering the structure. Because of bicchulite's structure it has a powdery texture, which leads to complications in obtaining information on the mineral's physical properties. Despite this problem, the color, specific gravity, and crystal size of bicchulite are known. Although bicchulite was only discovered about 40 years ago, technology has been rapidly advancing, allowing more accurate results to be made from experiments done today.
Davisite is an exceedingly rare mineral of the pyroxene group, with formula CaScAlSiO6. It is the scandium-dominant member. It stands for scandium-analogue of other pyroxene-group members, esseneite, grossmanite and kushiroite. Davisite is one of scarce minerals containing essential scandium.

Sodium tetrahydroxyborate is a salt (ionic compound) of with chemical formula NaH4BO4 or Na+[B(OH)4]−. It is one of several sodium borates. At room temperature it is a colorless transparent crystalline solid.
References
- 1 2 M. A. Simonov (1977): "The new mineral hexahydroborite, Ca[B(OH)4]2 · 2H2O". Zap. Vsesojuz. Mineral. Obshchest. SSSR, volume 106, issue 6, pages 691-697
- 1 2 3 4 5 6 7 8 9 M. A. Simonov (1979): "The new mineral hexahydroborite, Ca[B(OH)4]2 · 2H2O". International Geology Review, volume 21, issue 4, pages 491-496. doi : 10.1080/00206818209467082
- 1 2 3 4 5 6 N. A. Yamanova, E. Yu. Borovikova, and O. V. Dimitrova (2011): "Crystallization, crystal-structure refinement, and IR spectroscopy of a synthetic hexahydroborite analog". Crystallography Reports, volume 56, pages 1019–1024. doi : 10.1134/S1063774511060289
- ↑ Shoichi Kobayashi, Tamami Ando, Akiko Kanayama, Mitsuo Tanabe, Shigetomo Kishi, Isao Kusachi (2014): "Calciborite from the Fuka mine, Okayama Prefecture, Japan". Journal of Mineralogical and Petrological Sciences, volume 109, issue 1, pages 13-17. doi : 10.2465/jmps.130619
- 1 2 M. A. Simonov, N. A. Yamanova, E. V. Kazanskaya, Yu. K. Egorov-Tismenko, and N. V. Belov (1976) "Crystal structure of a new natural calcium borate,hexahydroborite CaB2O4 6H2O = Ca[B(OH)4 2 · 2H2O"]. Dokladi Akademii nauk SSSR, volume 228, pages 1337-1340. PACS numbers: 61.50.Qy
- 1 2 3 4 5 6 Isao Kusachi, Yasushi Takechi, Shoichi Kobayashi, Junji Yamakawa, Yoshihiro Nakamuta, Kyue-Hyung Lee, Shoji Motomizu (1999): "Hexahydroborite from Fuka, Okayama Prefecture, Japan". J-STAGE Mineralogical Journal, volume 21, issue 1, pags 9-14. doi : 10.2465/minerj.21.9