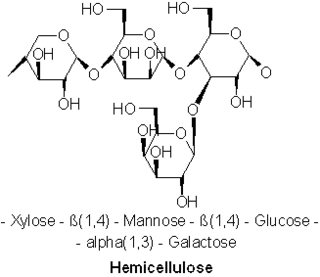
A hemicellulose is one of a number of heteropolymers, such as arabinoxylans, present along with cellulose in almost all terrestrial plant cell walls. Cellulose is crystalline, strong, and resistant to hydrolysis. Hemicelluloses are branched, shorter in length than cellulose, and also show a propensity to crystallize. They can be hydrolyzed by dilute acid or base as well as a myriad of hemicellulase enzymes.

Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH−.

Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity and do not rot easily. Chemically, lignins are polymers made by cross-linking phenolic precursors.

Sodium hypochlorite is an inorganic chemical compound with the formula NaOCl, comprising a sodium cation and a hypochlorite anion. It may also be viewed as the sodium salt of hypochlorous acid. The anhydrous compound is unstable and may decompose explosively. It can be crystallized as a pentahydrate NaOCl·5H
2O, a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated.

Pulp is a lignocellulosic fibrous material prepared by chemically or mechanically separating cellulose fibers from wood, fiber crops, waste paper, or rags. Mixed with water and other chemical or plant-based additives, pulp is the major raw material used in papermaking and the industrial production of other paper products.

The kraft process (also known as kraft pulping or sulfate process) is a process for conversion of wood into wood pulp, which consists of almost pure cellulose fibres, the main component of paper. The kraft process involves treatment of wood chips with a hot mixture of water, sodium hydroxide (NaOH), and sodium sulfide (Na2S), known as white liquor, that breaks the bonds that link lignin, hemicellulose, and cellulose. The technology entails several steps, both mechanical and chemical. It is the dominant method for producing paper. In some situations, the process has been controversial because kraft plants can release odorous products and in some situations produce substantial liquid wastes.

A pulp mill is a manufacturing facility that converts wood chips or other plant fiber sources into a thick fiber board which can be shipped to a paper mill for further processing. Pulp can be manufactured using mechanical, semi-chemical, or fully chemical methods. The finished product may be either bleached or non-bleached, depending on the customer requirements.
Resin acid refers to mixtures of several related carboxylic acids, primarily abietic acid, found in tree resins. Nearly all resin acids have the same basic skeleton: three fused rings having the empirical formula C19H29COOH. Resin acids are tacky, yellowish gums that are water-insoluble. They are used to produce soaps for diverse applications, but their use is being displaced increasingly by synthetic acids such as 2-ethylhexanoic acid or petroleum-derived naphthenic acids.
Resin soap is a mix of salts of resin acids. It is a yellow gelatinous pasty soap with use in bleaching and cleaning and as a compound of some varnishes. It also finds use in rubber industry.

Modified starch, also called starch derivatives, are prepared by physically, enzymatically, or chemically treating native starch to change its properties. Modified starches are used in practically all starch applications, such as in food products as a thickening agent, stabilizer or emulsifier; in pharmaceuticals as a disintegrant; or as binder in coated paper. They are also used in many other applications.

Bleach is the generic name for any chemical product that is used industrially or domestically to remove color (whitening) from a fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically to a dilute solution of sodium hypochlorite, also called "liquid bleach".
Bleaching of wood pulp is the chemical processing of wood pulp to lighten its color and whiten the pulp. The primary product of wood pulp is paper, for which whiteness is an important characteristic. These processes and chemistry are also applicable to the bleaching of non-wood pulps, such as those made from bamboo or kenaf.
The sulfite process produces wood pulp that is almost pure cellulose fibers by treating wood chips with solutions of sulfite and bisulfite ions. These chemicals cleave the bonds between the cellulose and lignin components of the lignocellulose. A variety of sulfite/bisulfite salts are used, including sodium (Na+), calcium (Ca2+), potassium (K+), magnesium (Mg2+), and ammonium (NH4+). The lignin is converted to lignosulfonates, which are soluble and can be separated from the cellulose fibers. For the production of cellulose, the sulfite process competes with the Kraft process which produces stronger fibers and is less environmentally costly.
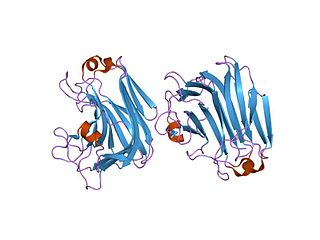
The enzyme mannuronate-specific alginate lyase catalyzes the degradation of alginate into various monosaccharide and polysaccharide products:
Deinking is the industrial process of removing printing ink from paperfibers of recycled paper to make deinked pulp.
Soda pulping is a chemical process for making wood pulp with sodium hydroxide as the cooking chemical. In the Soda-AQ process, anthraquinone (AQ) may be used as a pulping additive to decrease the carbohydrate degradation. The soda process gives pulp with lower tear strength than other chemical pulping processes, but has still limited use for easily-pulped materials like straw and some hardwoods.

The environmental effects of paper are significant, which has led to changes in industry and behaviour at both business and personal levels. With the use of modern technology such as the printing press and the highly mechanized harvesting of wood, disposable paper became a relatively cheap commodity, which led to a high level of consumption and waste. The rise in global environmental issues such as air and water pollution, climate change, overflowing landfills and clearcutting have all lead to increased government regulations. There is now a trend towards sustainability in the pulp and paper industry as it moves to reduce clear cutting, water use, greenhouse gas emissions, fossil fuel consumption and clean up its influence on local water supplies and air pollution.
White liquor is a strongly alkaline solution mainly of sodium hydroxide and sodium sulfide. It is used in the first stage of the Kraft process in which lignin and hemicellulose are separated from cellulose fiber for the production of pulp. The white liquor breaks the bonds between lignin and cellulose. It is called white liquor due to its white opaque colour.
In industrial paper-making processes, organosolv is a pulping technique that uses an organic solvent to solubilise lignin and hemicellulose. It has been considered in the context of both pulp and paper manufacture and biorefining for subsequent conversion of cellulose to fuel ethanol. The process was invented by Theodor Kleinert in 1968 as an environmentally benign alternative to kraft pulping.

Paper chemicals designate a group of chemicals that are used for paper manufacturing, or modify the properties of paper. These chemicals can be used to alter the paper in many ways, including changing its color and brightness, or by increasing its strength and resistance to water. The chemicals can be defined on basis of their usage in the process.













