
In humans, vocal cords, also known as vocal folds or voice reeds, are folds of throat tissues that are key in creating sounds through vocalization. The size of vocal cords affects the pitch of voice. Open when breathing and vibrating for speech or singing, the folds are controlled via the recurrent laryngeal branch of the vagus nerve. They are composed of twin infoldings of mucous membrane stretched horizontally, from back to front, across the larynx. They vibrate, modulating the flow of air being expelled from the lungs during phonation.

The larynx, commonly called the voice box, is an organ in the top of the neck involved in breathing, producing sound and protecting the trachea against food aspiration. The opening of larynx into pharynx known as the laryngeal inlet is about 4–5 centimeters in diameter. The larynx houses the vocal cords, and manipulates pitch and volume, which is essential for phonation. It is situated just below where the tract of the pharynx splits into the trachea and the esophagus. The word ʻlarynxʼ comes from the Ancient Greek word lárunx ʻlarynx, gullet, throat.ʼ

Cartilage is a resilient and smooth type of connective tissue. In tetrapods, it covers and protects the ends of long bones at the joints as articular cartilage, and is a structural component of many body parts including the rib cage, the neck and the bronchial tubes, and the intervertebral discs. In other taxa, such as chondrichthyans, but also in cyclostomes, it may constitute a much greater proportion of the skeleton. It is not as hard and rigid as bone, but it is much stiffer and much less flexible than muscle. The matrix of cartilage is made up of glycosaminoglycans, proteoglycans, collagen fibers and, sometimes, elastin.

Reinke's edema is the swelling of the vocal cords due to fluid (edema) collected within the Reinke's space. First identified by the German anatomist Friedrich B. Reinke in 1895, the Reinke's space is a gelatinous layer of the vocal cord located underneath the outer cells of the vocal cord. When a person speaks, the Reinke's space vibrates to allow for sound to be produced (phonation). The Reinke's space is sometimes referred to as the superficial lamina propria.
Articles related to anatomy include:
Connective tissue is one of the four primary types of animal tissue, along with epithelial tissue, muscle tissue, and nervous tissue. It develops from the mesenchyme derived from the mesoderm the middle embryonic germ layer. Connective tissue is found in between other tissues everywhere in the body, including the nervous system. The three meninges, membranes that envelop the brain and spinal cord are composed of connective tissue. Most types of connective tissue consists of three main components: elastic and collagen fibers, ground substance, and cells. Blood, and lymph are classed as specialized fluid connective tissues that do not contain fiber. All are immersed in the body water. The cells of connective tissue include fibroblasts, adipocytes, macrophages, mast cells and leucocytes.

The epiglottis is a leaf-shaped flap in the throat that prevents food and water from entering the trachea and the lungs. It stays open during breathing, allowing air into the larynx. During swallowing, it closes to prevent aspiration of food into the lungs, forcing the swallowed liquids or food to go along the oesophagus toward the stomach instead. It is thus the valve that diverts passage to either the trachea or the oesophagus.

The nasal cavity is a large, air-filled space above and behind the nose in the middle of the face. The nasal septum divides the cavity into two cavities, also known as fossae. Each cavity is the continuation of one of the two nostrils. The nasal cavity is the uppermost part of the respiratory system and provides the nasal passage for inhaled air from the nostrils to the nasopharynx and rest of the respiratory tract.
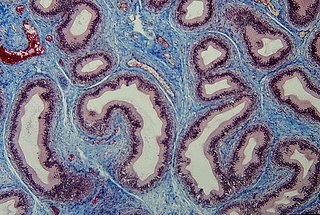
The lamina propria is a thin layer of connective tissue that forms part of the moist linings known as mucous membranes or mucosae, which line various tubes in the body, such as the respiratory tract, the gastrointestinal tract, and the urogenital tract.
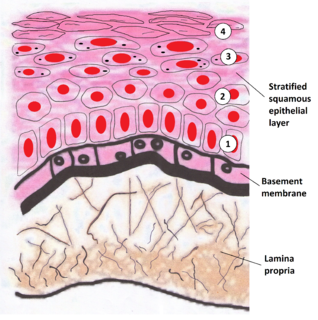
The basal lamina is a layer of extracellular matrix secreted by the epithelial cells, on which the epithelium sits. It is often incorrectly referred to as the basement membrane, though it does constitute a portion of the basement membrane. The basal lamina is visible only with the electron microscope, where it appears as an electron-dense layer that is 20–100 nm thick.

Loose connective tissue, sometimes called areolar tissue, is a cellular connective tissue with thin and relatively sparse collagen fibers. Its ground substance occupies more volume than the fibers do. It has a viscous to gel-like consistency and plays an important role in the diffusion of oxygen and nutrients from the capillaries that course through this connective tissue as well as in the diffusion of carbon dioxide and metabolic wastes back to the vessels. Moreover, loose connective tissue is primarily located beneath the epithelia that cover the body surfaces and line the internal surfaces of the body. It is also associated with the epithelium of glands and surrounds the smallest blood vessels. This tissue is thus the initial site where pathogenic agents, such as bacteria that have breached an epithelial surface, are challenged and destroyed by cells of the immune system.

Elastic cartilage, fibroelastic cartilage or yellow fibrocartilage is a type of cartilage present in the pinnae (auricles) of the ear giving it shape, provides shape for the lateral region of the external auditory meatus, medial part of the auditory canal Eustachian tube, corniculate and cuneiform laryneal cartilages, and the epiglottis. It contains elastic fiber networks and collagen type II fibers. The principal protein is elastin.
Lamina is a general anatomical term meaning "plate" or "layer". It is used in both gross anatomy and microscopic anatomy to describe structures.
The oral mucosa is the mucous membrane lining the inside of the mouth. It comprises stratified squamous epithelium, termed "oral epithelium", and an underlying connective tissue termed lamina propria. The oral cavity has sometimes been described as a mirror that reflects the health of the individual. Changes indicative of disease are seen as alterations in the oral mucosa lining the mouth, which can reveal systemic conditions, such as diabetes or vitamin deficiency, or the local effects of chronic tobacco or alcohol use. The oral mucosa tends to heal faster and with less scar formation compared to the skin. The underlying mechanism remains unknown, but research suggests that extracellular vesicles might be involved.

The thyroarytenoid muscle is a broad, thin muscle that forms the body of the vocal fold and that supports the wall of the ventricle and its appendix. It functions to shorten the vocal folds.
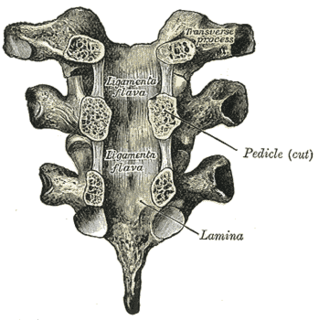
The ligamenta flava are a series of ligaments that connect the ventral parts of the laminae of adjacent vertebrae. They help to preserve upright posture, preventing hyperflexion, and ensuring that the vertebral column straightens after flexion. Hypertrophy can cause spinal stenosis.
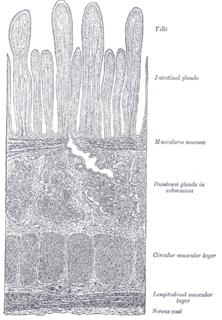
The lamina muscularis mucosae is a thin layer of muscle of the gastrointestinal tract, located outside the lamina propria, and separating it from the submucosa. It is present in a continuous fashion from the esophagus to the upper rectum. A discontinuous muscularis mucosae–like muscle layer is present in the urinary tract, from the renal pelvis to the bladder; as it is discontinuous, it should not be regarded as a true muscularis mucosae.
Tissue engineering of oral mucosa combines cells, materials and engineering to produce a three-dimensional reconstruction of oral mucosa. It is meant to simulate the real anatomical structure and function of oral mucosa. Tissue engineered oral mucosa shows promise for clinical use, such as the replacement of soft tissue defects in the oral cavity. These defects can be divided into two major categories: the gingival recessions which are tooth-related defects, and the non tooth-related defects. Non tooth-related defects can be the result of trauma, chronic infection or defects caused by tumor resection or ablation. Common approaches for replacing damaged oral mucosa are the use of autologous grafts and cultured epithelial sheets.

The gastrointestinal wall of the gastrointestinal tract is made up of four layers of specialised tissue. From the inner cavity of the gut outwards, these are:
- Mucosa
- Submucosa
- Muscular layer
- Serosa or adventitia
Thyroplasty is a phonosurgical technique designed to improve the voice by altering the thyroid cartilage of the larynx, which houses the vocal cords in order to change the position or the length of the vocal cords.















