
Dynamite is an explosive made of nitroglycerin, sorbents, and stabilizers. It was invented by the Swedish chemist and engineer Alfred Nobel in Geesthacht, Northern Germany, and was patented in 1867. It rapidly gained wide-scale use as a more robust alternative to the traditional black powder explosives. It allows the use of nitroglycerine's favorable explosive properties while greatly reducing its risk of accidental detonation.

An explosive is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances.

Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.

Trinitrotoluene, more commonly known as TNT, more specifically 2,4,6-trinitrotoluene, and by its preferred IUPAC name 2-methyl-1,3,5-trinitrobenzene, is a chemical compound with the formula C6H2(NO2)3CH3. TNT is occasionally used as a reagent in chemical synthesis, but it is best known as an explosive material with convenient handling properties. The explosive yield of TNT is considered to be the standard comparative convention of bombs and asteroid impacts. In chemistry, TNT is used to generate charge transfer salts.

Hydrazine is an inorganic compound with the chemical formula N2H4. It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine hydrate.
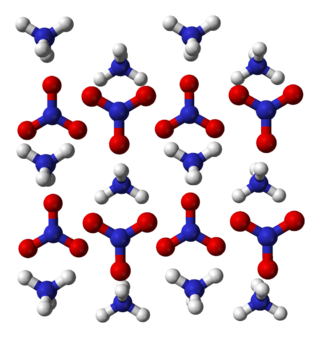
Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer.

ANFO ( AN-foh) (or AN/FO, for ammonium nitrate/fuel oil) is a widely used bulk industrial explosive. It consists of 94% porous prilled ammonium nitrate (NH4NO3) (AN), which acts as the oxidizing agent and absorbent for the fuel, and 6% number 2 fuel oil (FO). The use of ANFO originated in the 1950s.
Nitromethane, sometimes shortened to simply "nitro", is an organic compound with the chemical formula CH
3NO
2. It is the simplest organic nitro compound. It is a polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions, as a reaction medium, and as a cleaning solvent. As an intermediate in organic synthesis, it is used widely in the manufacture of pesticides, explosives, fibers, and coatings. Nitromethane is used as a fuel additive in various motorsports and hobbies, e.g. Top Fuel drag racing and miniature internal combustion engines in radio control, control line and free flight model aircraft.
A propellant is a mass that is expelled or expanded in such a way as to create a thrust or another motive force in accordance with Newton's third law of motion, and "propel" a vehicle, projectile, or fluid payload. In vehicles, the engine that expels the propellant is called a reaction engine. Although technically a propellant is the reaction mass used to create thrust, the term "propellant" is often used to describe a substance which contains both the reaction mass and the fuel that holds the energy used to accelerate the reaction mass. For example, the term "propellant" is often used in chemical rocket design to describe a combined fuel/propellant, although the propellants should not be confused with the fuel that is used by an engine to produce the energy that expels the propellant. Even though the byproducts of substances used as fuel are also often used as a reaction mass to create the thrust, such as with a chemical rocket engine, propellant and fuel are two distinct concepts.

Methyl nitrate is the methyl ester of nitric acid and has the chemical formula CH3NO3. It is a colourless explosive volatile liquid.
Astrolite is the trade name of a family of explosives, invented by chemist Gerald Hurst in the 1960s during his employment with the Atlas Powder Company. The Astrolite family consists of two compounds, Astrolite G and Astrolite A. Both are two-part liquid-state high explosive mixtures, composed of ammonium nitrate oxidizer and hydrazine rocket fuel. The explosives were extensively studied, manufactured, and used in many countries because of their advantages of high energy, excellent performance, and wide application. They still find some use in commercial and civil blasting applications, but have mostly been superseded by cheaper and safer compounds, largely due to the expense and exceptionally poisonous nature of the hydrazine component.

The Oppau explosion occurred on September 21, 1921, when approximately 4,500 metric tons of a mixture of ammonium sulfate and ammonium nitrate fertilizer stored in a tower silo exploded at a BASF plant in Oppau, now part of Ludwigshafen, Germany, killing 500–600 people and injuring about 2,000 more.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.

Erythritol tetranitrate (ETN) is an explosive compound chemically similar to PETN, though it is thought to be slightly more sensitive to friction and impact.

Urea nitrate is a fertilizer-based high explosive that has been used in improvised explosive devices in Afghanistan, Pakistan, Iraq, and various terrorist acts elsewhere in the world such as in the 1993 World Trade Center bombings. It has a destructive power similar to better-known ammonium nitrate explosives, with a velocity of detonation between 3,400 m/s (11,155 ft/s) and 4,700 m/s (15,420 ft/s). It has chemical formula of CH5N3O4 or (NH2)2COHNO3.

Carbohydrazide is the chemical compound with the formula OC(N2H3)2. It appears as a white solid that is soluble in water, but not in many organic solvents, such as ethanol, ether or benzene. It decomposes upon melting. A number of carbazides are known where one or more N-H groups are replaced by other substituents. They occur widely in the drugs, herbicides, plant growth regulators, and dyestuffs.

Ammonium dinitramide (ADN) is the ammonium salt of dinitraminic acid. ADN decomposes under heat to leave only nitrogen, oxygen, and water. The ions are the ammonium ion NH4+ and the dinitramide N(NO2)2−.
Bulk loaded liquid propellants are an artillery technology that was pursued at the U.S. Army Research Laboratory and U.S. Naval Weapons Center from the 1950s through the 1990s. The advantages would be simpler guns and a wider range of tactical and logistic options. Better accuracy and tactical flexibility would theoretically come from standard shells with varying propellant loads, and logistic simplification by eliminating varying powder loads.

Nickel hydrazine nitrate (NHN), (chemical formula: [Ni(N2H4)3](NO3)2 is an energetic material having explosive properties in between that of primary explosive and a secondary explosive. It is a salt of a coordination compound of nickel with a reaction equation of 3N2H4·H2O + Ni(NO3)2 →〔Ni(N2H4)3〕(NO3)2 + 3H2O














