Related Research Articles

A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding.

A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.

The noble gases are the naturally occurring members of group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Under standard conditions, these elements are odorless, colorless, monatomic gases with very low chemical reactivity and cryogenic boiling points.
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the cation [H3O]+, also written as H3O+, the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved in water, as Arrhenius acid molecules in solution give up a proton (a positive hydrogen ion, H+) to the surrounding water molecules (H2O). In fact, acids must be surrounded by more than a single water molecule in order to ionize, yielding aqueous H+ and conjugate base. Three main structures for the aqueous proton have garnered experimental support: the Eigen cation, which is a tetrahydrate, H3O+(H2O)3, the Zundel cation, which is a symmetric dihydrate, H+(H2O)2, and the Stoyanov cation, an expanded Zundel cation, which is a hexahydrate: H+(H2O)2(H2O)4. Spectroscopic evidence from well-defined IR spectra overwhelmingly supports the Stoyanov cation as the predominant form. For this reason, it has been suggested that wherever possible, the symbol H+(aq) should be used instead of the hydronium ion.

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.
Penning ionization is a form of chemi-ionization, an ionization process involving reactions between neutral atoms or molecules. The Penning effect is put to practical use in applications such as gas-discharge neon lamps and fluorescent lamps, where the lamp is filled with a penning mixture to improve the electrical characteristics of the lamps.
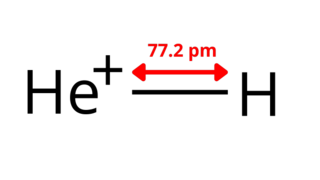
The helium hydride ion, hydridohelium(1+) ion, or helonium is a cation (positively charged ion) with chemical formula HeH+. It consists of a helium atom bonded to a hydrogen atom, with one electron removed. It can also be viewed as protonated helium. It is the lightest heteronuclear ion, and is believed to be the first compound formed in the Universe after the Big Bang.
Mass spectral interpretation is the method employed to identify the chemical formula, characteristic fragment patterns and possible fragment ions from the mass spectra. Mass spectra is a plot of relative abundance against mass-to-charge ratio. It is commonly used for the identification of organic compounds from electron ionization mass spectrometry. Organic chemists obtain mass spectra of chemical compounds as part of structure elucidation and the analysis is part of many organic chemistry curricula.

The dihydrogen cation or hydrogen molecular ion is a cation with formula H+
2. It consists of two hydrogen nuclei (protons), each sharing a single electron. It is the simplest molecular ion.

An ion is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons.
Photoelectrochemical processes are processes in photoelectrochemistry; they usually involve transforming light into other forms of energy. These processes apply to photochemistry, optically pumped lasers, sensitized solar cells, luminescence, and photochromism.
Triatomic hydrogen or H3 is an unstable triatomic molecule containing only hydrogen. Since this molecule contains only three atoms of hydrogen it is the simplest triatomic molecule and it is relatively simple to numerically solve the quantum mechanics description of the particles. Being unstable the molecule breaks up in under a millionth of a second. Its fleeting lifetime makes it rare, but it is quite commonly formed and destroyed in the universe thanks to the commonness of the trihydrogen cation. The infrared spectrum of H3 due to vibration and rotation is very similar to that of the ion, H+
3. In the early universe this ability to emit infrared light allowed the primordial hydrogen and helium gas to cool down so as to form stars.

Chromium(I) hydride, systematically named chromium hydride, is an inorganic compound with the chemical formula (CrH)
n. It occurs naturally in some kinds of stars where it has been detected by its spectrum. However, molecular chromium(I) hydride with the formula CrH has been isolated in solid gas matrices. The molecular hydride is very reactive. As such the compound is not well characterised, although many of its properties have been calculated via computational chemistry.
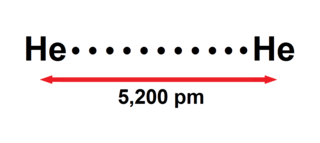
The helium dimer is a van der Waals molecule with formula He2 consisting of two helium atoms. This chemical is the largest diatomic molecule—a molecule consisting of two atoms bonded together. The bond that holds this dimer together is so weak that it will break if the molecule rotates, or vibrates too much. It can only exist at very low cryogenic temperatures.
Helium is the smallest and the lightest noble gas and one of the most unreactive elements, so it was commonly considered that helium compounds cannot exist at all, or at least under normal conditions. Helium's first ionization energy of 24.57 eV is the highest of any element. Helium has a complete shell of electrons, and in this form the atom does not readily accept any extra electrons nor join with anything to make covalent compounds. The electron affinity is 0.080 eV, which is very close to zero. The helium atom is small with the radius of the outer electron shell at 0.29 Å. Helium is a very hard atom with a Pearson hardness of 12.3 eV. It has the lowest polarizability of any kind of atom, however, very weak van der Waals forces exist between helium and other atoms. This force may exceed repulsive forces, so at extremely low temperatures helium may form van der Waals molecules. Helium has the lowest boiling point of any known substance.
Neon compounds are chemical compounds containing the element neon (Ne) with other molecules or elements from the periodic table. Compounds of the noble gas neon were believed not to exist, but there are now known to be molecular ions containing neon, as well as temporary excited neon-containing molecules called excimers. Several neutral neon molecules have also been predicted to be stable, but are yet to be discovered in nature. Neon has been shown to crystallize with other substances and form clathrates or Van der Waals solids.
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO.
The magnesium argide ion, MgAr+ is an ion composed of one ionised magnesium atom, Mg+ and an argon atom. It is important in inductively coupled plasma mass spectrometry and in the study of the field around the magnesium ion. The ionization potential of magnesium is lower than the first excitation state of argon, so the positive charge in MgAr+ will reside on the magnesium atom. Neutral MgAr molecules can also exist in an excited state.
In chemistry, the decay technique is a method to generate chemical species such as radicals, carbocations, and other potentially unstable covalent structures by radioactive decay of other compounds. For example, decay of a tritium-labeled molecule yields an ionized helium atom, which might then break off to leave a cationic molecular fragment.
References
- ↑ Sattler, Klaus D. (2010). "Electron Impact Ionization of Hydrogen Clusters Embedded in Helium". Clusters and Fullerenes. Handbook of Nanophysics. CRC Press. pp. 20–15–20–17. ISBN 978-1-4200-7554-0.
- ↑ Ching Yeh Lin; Andrew T.B. Gilbert; Mark A. Walter (6 May 2011). "Interstellar Solid Hydrogen". The Astrophysical Journal. 736 (2): 91. arXiv: 1105.1861 . Bibcode:2011ApJ...736...91L. doi:10.1088/0004-637X/736/2/91. S2CID 50907532.
- ↑ Takayuki Kumada; Yuta Shimizu; Takahiro Ushida; Jun Kumagai (October–December 2008). "H atom, e−, and H+
6 ions produced in irradiated solid hydrogens: An electron spin resonance study". Radiation Physics and Chemistry. 77 (10–12). Elsevier: 1318–1322. Bibcode:2008RaPC...77.1318K. doi:10.1016/j.radphyschem.2008.05.026. - ↑ J. Kumagai; H. Inagaki; S. Kariya; T. Ushida; Y. Shimizu; T. Kumada (14 July 2007). "Electron spin resonance study on H+
6, H
5D+
, H
4D+
2, and H
2D+
4 in solid parahydrogen". J Chem Phys. 127 (2): 024505. Bibcode:2007JChPh.127b4505K. doi:10.1063/1.2748046. PMID 17640135. - ↑ R. Clampitt, L. Gowland; Gowland, L. (August 1969). "Clustering of Cold Hydrogen Gas on Protons". Nature. 223 (5208): 815–816. Bibcode:1969Natur.223..815C. doi:10.1038/223815a0. S2CID 4172620.
- ↑ R. Clampitt; D. K. Jefferies (11 April 1970). "Ion Clusters". Nature. 226 (5241): 141–142. Bibcode:1970Natur.226..141C. doi:10.1038/226141a0. PMID 16057136. S2CID 43445356.
- ↑ Hiroka, K. (1987). "A determination of the stabilities of H+
3(H
2)
n with n = 1−9 from measurements of the gas-phase ion equilibria H+
3(H
2)
n−1+H
2 = H+
3(H
2)
n". The Journal of Chemical Physics. 87 (7). American Institute of Physics: 4048–4055. Bibcode:1987JChPh..87.4048H. doi:10.1063/1.452909. ISSN 0021-9606. - 1 2 3 4 5 6 Bae, Young K.; Phillip C. Cosby (September 1990). "Ionic Solid Hydrogen Fuel: Production and Properties of Hydrogen Ion and Energetic Neutral Clusters" (PDF). Astronautics Laboratory. Archived from the original on October 8, 2012. Retrieved 17 June 2011.
- 1 2 3 Kirchner, Nicholas J.; Michael T. Bowers (1987). "An experimental study of the formation and reactivity of ionic hydrogen clusters: The first observation and characterization of the even clusters H+
4, H+
6, H+
8, and H+
10". Journal of Chemical Physics. 86 (3): 1301–1310. Bibcode:1987JChPh..86.1301K. doi:10.1063/1.452219. - 1 2 Kurosaki, Yuzuru; Toshiyuki. Takayanagi (21 August 1998). "A direct isomerization path for the H+
6 cluster. An ab initio molecular orbital study". Chemical Physics Letters. 293 (1–2). Elsevier Science B.V.: 59–64. Bibcode:1998CPL...293...59K. doi:10.1016/S0009-2614(98)00721-0. - ↑ Qiang Hao; Andrew C. Simmonett; Yukio Yamaguchi; Fang De-Cai; Henry F. Schaeffer (23 October 2009). "Structures and Energetics of H+
6 Clusters". The Journal of Physical Chemistry A. 113 (48). Washington DC: American Chemical Society: 13608–13620. Bibcode:2009JPCA..11313608H. doi:10.1021/jp905928u. ISSN 1089-5639. PMID 19852448. - ↑ Takayuki Kumada; Hiroto Tachikawa; Toshiyuki Takayanagi (2005). "H+
6 in irradiated solid para-hydrogen and its decay dynamics: reinvestigation of quartet electron paramagnetic resonance lines assigned to H−
2". Physical Chemistry Chemical Physics. 7 (5): 776–784. Bibcode:2005PCCP....7..776K. doi:10.1039/b415179h. ISSN 1463-9076. PMID 19791361. - ↑ Ekinci, Y; E. L. Knuth; J. P. Toennies (5 October 2006). "A mass and time-of-flight spectroscopy study of the formation of clusters in free-jet expansions of normal D
2". Journal of Chemical Physics. 125 (13): 133409–133420. Bibcode:2006JChPh.125m3409E. doi:10.1063/1.2217942. PMID 17029483.