Related Research Articles

Hydrogen is a chemical element; it has symbol H and atomic number 1. It is the lightest element and, at standard conditions, is a gas of diatomic molecules with the formula H2. It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen each atom has one proton, one electron, and no neutrons.

In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. These surrounded solute particles then move away from the solid solute and out into the solution. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when one of the solvents is water.

A pipeline is a system of pipes for long-distance transportation of a liquid or gas, typically to a market area for consumption. The latest data from 2014 gives a total of slightly less than 2,175,000 miles (3,500,000 km) of pipeline in 120 countries around the world. The United States had 65%, Russia had 8%, and Canada had 3%, thus 76% of all pipeline were in these three countries.

Thermite is a pyrotechnic composition of metal powder and metal oxide. When ignited by heat or chemical reaction, thermite undergoes an exothermic reduction-oxidation (redox) reaction. Most varieties are not explosive, but can create brief bursts of heat and high temperature in a small area. Its form of action is similar to that of other fuel-oxidizer mixtures, such as black powder.

Liquid hydrogen (H2(l)) is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecular H2 form.
A substance is pyrophoric if it ignites spontaneously in air at or below 54 °C (129 °F) or within 5 minutes after coming into contact with air. Examples are organolithium compounds and triethylborane. Pyrophoric materials are often water-reactive as well and will ignite when they contact water or humid air. They can be handled safely in atmospheres of argon or nitrogen. Class D fire extinguishers are designated for use in fires involving pyrophoric materials. A related concept is hypergolicity, in which two compounds spontaneously ignite when mixed.
A propellant is a mass that is expelled or expanded in such a way as to create a thrust or another motive force in accordance with Newton's third law of motion, and "propel" a vehicle, projectile, or fluid payload. In vehicles, the engine that expels the propellant is called a reaction engine. Although technically a propellant is the reaction mass used to create thrust, the term "propellant" is often used to describe a substance which contains both the reaction mass and the fuel that holds the energy used to accelerate the reaction mass. For example, the term "propellant" is often used in chemical rocket design to describe a combined fuel/propellant, although the propellants should not be confused with the fuel that is used by an engine to produce the energy that expels the propellant. Even though the byproducts of substances used as fuel are also often used as a reaction mass to create the thrust, such as with a chemical rocket engine, propellant and fuel are two distinct concepts.

A liquid-propellant rocket or liquid rocket utilizes a rocket engine that uses liquid propellants. Gaseous propellants may also be used but are not common because of their low density and difficulty with common pumping methods. Liquids are desirable because they have a reasonably high density and high specific impulse (Isp). This allows the volume of the propellant tanks to be relatively low. The rocket propellants are usually pumped into the combustion chamber with a lightweight centrifugal turbopump, although some aerospace companies have found ways to use electric pumps with batteries, allowing the propellants to be kept under low pressure. This permits the use of low-mass propellant tanks that do not need to resist the high pressures needed to store significant amounts of gasses, resulting in a low mass ratio for the rocket.
A supercritical fluid (SCF) is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist, but below the pressure required to compress it into a solid. It can effuse through porous solids like a gas, overcoming the mass transfer limitations that slow liquid transport through such materials. SCF are much superior to gases in their ability to dissolve materials like liquids or solids. Also, near the critical point, small changes in pressure or temperature result in large changes in density, allowing many properties of a supercritical fluid to be "fine-tuned".

The hydrogen economy is an umbrella term that draws together the roles hydrogen can play alongside renewable electricity to decarbonize those sectors and activities which may be technically difficult to decarbonize through other means, or where cheaper and more energy-efficient clean solutions are not available. In this context, hydrogen economy encompasses hydrogen's production through to end-uses in ways that substantively contribute to avoiding the use of fossil fuels and mitigating greenhouse gas emissions.

Fluidization is a process similar to liquefaction whereby a granular material is converted from a static solid-like state to a dynamic fluid-like state. This process occurs when a fluid is passed up through the granular material.

Industrial gases are the gaseous materials that are manufactured for use in industry. The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene, although many other gases and mixtures are also available in gas cylinders. The industry producing these gases is also known as industrial gas, which is seen as also encompassing the supply of equipment and technology to produce and use the gases. Their production is a part of the wider chemical Industry.

Electrolysis of water is using electricity to split water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, but must be kept apart from the oxygen as the mixture would be extremely explosive. Separately pressurised into convenient 'tanks' or 'gas bottles', hydrogen can be used for oxyhydrogen welding and other applications, as the hydrogen / oxygen flame can reach approximately 2,800°C.
Hydrogen production is the family of industrial methods for generating hydrogen gas. There are four main sources for the commercial production of hydrogen: natural gas, oil, coal, and electrolysis of water; which account for 48%, 30%, 18% and 4% of the world's hydrogen production respectively. Fossil fuels are the dominant source of industrial hydrogen. As of 2020, the majority of hydrogen (~95%) is produced by steam reforming of natural gas and other light hydrocarbons, partial oxidation of heavier hydrocarbons, and coal gasification. Other methods of hydrogen production include biomass gasification and methane pyrolysis. Methane pyrolysis and water electrolysis can use any source of electricity including renewable energy.

Plasma-enhanced chemical vapor deposition (PECVD) is a chemical vapor deposition process used to deposit thin films from a gas state (vapor) to a solid state on a substrate. Chemical reactions are involved in the process, which occur after creation of a plasma of the reacting gases. The plasma is generally created by radio frequency (RF) frequency or direct current (DC) discharge between two electrodes, the space between which is filled with the reacting gases.

Hydrogen safety covers the safe production, handling and use of hydrogen, particularly hydrogen gas fuel and liquid hydrogen.
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.

Water is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an inherent hint of blue. It is by far the most studied chemical compound and is described as the "universal solvent" and the "solvent of life". It is the most abundant substance on the surface of Earth and the only common substance to exist as a solid, liquid, and gas on Earth's surface. It is also the third most abundant molecule in the universe.

Rocket propellant is the reaction mass of a rocket. This reaction mass is ejected at the highest achievable velocity from a rocket engine to produce thrust. The energy required can either come from the propellants themselves, as with a chemical rocket, or from an external source, as with ion engines.
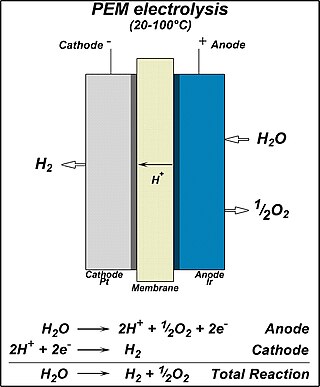
Proton exchange membrane(PEM) electrolysis is the electrolysis of water in a cell equipped with a solid polymer electrolyte (SPE) that is responsible for the conduction of protons, separation of product gases, and electrical insulation of the electrodes. The PEM electrolyzer was introduced to overcome the issues of partial load, low current density, and low pressure operation currently plaguing the alkaline electrolyzer. It involves a proton-exchange membrane.
References
- 1 2 "Hydrogen Delivery". Energy.gov. Retrieved 2022-08-06.
- ↑ Blain, Loz (2022-07-22). "Another hydrogen transport powder emerges, promising double the density". New Atlas. Retrieved 2022-08-05.
- ↑ Blain, Loz (2022-07-19). "Mechanochemical breakthrough unlocks cheap, safe, powdered hydrogen". New Atlas. Retrieved 2022-08-05.
- 1 2 3 4 Gerboni, R. (2016). "Hydrogen Transportation - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2022-08-06.
- ↑ Read "Emergency and Continuous Exposure Guidance Levels for Selected Submarine Contaminants: Volume 2" at NAP.edu. 2008. doi:10.17226/12032. ISBN 978-0-309-11273-4.