
Miso is a traditional Japanese seasoning. It is a thick paste produced by fermenting soybeans with salt and kōji and sometimes rice, barley, seaweed, or other ingredients. It is used for sauces and spreads, pickling vegetables, fish, or meats, and mixing with dashi soup stock to serve as miso soup, a Japanese culinary staple. Miso is high in protein and rich in vitamins and minerals, and it played an important nutritional role in feudal Japan. Miso is still widely used in both traditional and modern cooking in Japan and has been gaining worldwide interest.

Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the digestive system of many vertebrates, where it hydrolyzes proteins. Trypsin is formed in the small intestine when its proenzyme form, the trypsinogen produced by the pancreas, is activated. Trypsin cuts peptide chains mainly at the carboxyl side of the amino acids lysine or arginine. It is used for numerous biotechnological processes. The process is commonly referred to as trypsinogen proteolysis or trypsinization, and proteins that have been digested/treated with trypsin are said to have been trypsinized. Trypsin was discovered in 1876 by Wilhelm Kühne and was named from the Ancient Greek word for rubbing since it was first isolated by rubbing the pancreas with glycerin.

Yeast extracts consist of the cell contents of yeast without the cell walls; they are used as food additives or flavorings, or as nutrients for bacterial culture media. They are often used to create savory flavors and umami taste sensations and can be found in a large variety of packaged food including frozen meals, crackers, snack foods, gravy, stock and more. They are rich in B vitamins. Yeast extracts and fermented foods contain glutamic acid, an amino acid which adds an umami flavor. Glutamic acid is found in meat, cheese, fungi and vegetables—such as broccoli and tomatoes. A number of other substances found in yeast extract provide aromas, some meat-like, when allowed to react under heat.

Soy sauce is a liquid condiment of Chinese origin, traditionally made from a fermented paste of soybeans, roasted grain, brine, and Aspergillus oryzae or Aspergillus sojae molds. It is recognized for its pronounced umami taste.

Monosodium glutamate (MSG), also known as sodium glutamate, is a sodium salt of glutamic acid. MSG is found naturally in some foods including tomatoes and cheese in this glutamic acid form. MSG is used in cooking as a flavor enhancer with a savory taste that intensifies the meaty, savory flavor of food, as naturally occurring glutamate does in foods such as stews and meat soups.

The Maillard reaction is a chemical reaction between amino acids and reducing sugars to create melanoidins, the compounds which give browned food its distinctive flavor. Seared steaks, fried dumplings, cookies and other kinds of biscuits, breads, toasted marshmallows, and many other foods undergo this reaction. It is named after French chemist Louis Camille Maillard, who first described it in 1912 while attempting to reproduce biological protein synthesis. The reaction is a form of non-enzymatic browning which typically proceeds rapidly from around 140 to 165 °C. Many recipes call for an oven temperature high enough to ensure that a Maillard reaction occurs. At higher temperatures, caramelization and subsequently pyrolysis become more pronounced.

A meat alternative or meat substitute, is a food product made from vegetarian or vegan ingredients, eaten as a replacement for meat. Meat alternatives typically approximate qualities of specific types of meat, such as mouthfeel, flavor, appearance, or chemical characteristics. Plant- and fungus-based substitutes are frequently made with soy, but may also be made from wheat gluten as in seitan, pea protein as in the Beyond Burger, or mycoprotein as in Quorn. Alternative protein foods can also be made by precision fermentation, where single cell organisms such as yeast produce specific proteins using a carbon source; as well as cultivated or laboratory grown, based on tissue engineering techniques.
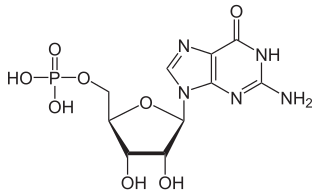
Guanosine monophosphate (GMP), also known as 5′-guanidylic acid or guanylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid with the nucleoside guanosine. GMP consists of the phosphate group, the pentose sugar ribose, and the nucleobase guanine; hence it is a ribonucleotide monophosphate. Guanosine monophosphate is commercially produced by microbial fermentation.
Hydrolyzed protein is a solution derived from the hydrolysis of a protein into its component amino acids and peptides. While many means of achieving this exist, most common is prolonged heating with hydrochloric acid, sometimes with an enzyme such as pancreatic protease to simulate the naturally occurring hydrolytic process.

Fermented tofu is a Chinese condiment consisting of a form of processed, preserved tofu used in East Asian cuisine. The ingredients typically are soybeans, salt, rice wine and sesame oil or vinegar. In mainland China the product is often freshly distributed. In overseas Chinese communities living in Southeast Asia, commercially packaged versions are often sold in jars containing blocks 2- to 4-cm square by 1 to 2 cm thick soaked in brine with select flavorings.

Soy allergy is a type of food allergy. It is a hypersensitivity to ingesting compounds in soy, causing an overreaction of the immune system, typically with physical symptoms, such as gastrointestinal discomfort, respiratory distress, or a skin reaction. Soy is among the eight most common foods inducing allergic reactions in children and adults. It has a prevalence of about 0.3% in the general population.

Wheat allergy is an allergy to wheat which typically presents itself as a food allergy, but can also be a contact allergy resulting from occupational exposure. Like all allergies, wheat allergy involves immunoglobulin E and mast cell response. Typically the allergy is limited to the seed storage proteins of wheat. Some reactions are restricted to wheat proteins, while others can react across many varieties of seeds and other plant tissues. Wheat allergy is rare. Prevalence in adults was found to be 0.21% in a 2012 study in Japan.

Glutamate flavoring is the generic name for flavor-enhancing compounds based on glutamic acid and its salts (glutamates). These compounds provide an umami (savory) taste to food.

3-MCPD (3-monochloropropane-1,2-diol or 3-chloropropane-1,2-diol) is an organic chemical compound with the formula HOCH2CH(OH)CH2Cl. It is a colorless liquid. It is a versatile multifunctional building block. The compound has attracted attention as the most common member of chemical food contaminants known as chloropropanols. It is suspected to be carcinogenic in humans.
Rice protein is a vegan protein isolate made from rice. It is often used as an alternative to the more common whey and soy protein isolates. To make it, brown rice is treated with enzymes that cause carbohydrates to separate from proteins. The resulting protein powder may then be flavored, and consumed with water, milk, or added to smoothies or health shakes.
Caricain is an enzyme. This enzyme catalyses the following chemical reaction: Hydrolysis of proteins with broad specificity for peptide bonds, similar to those of papain and chymopapain
Fish protein powder (FPP) describes a food grade powder product designated primarily for human consumption applications. It differs significantly from fish meal products which are designated for animal feed applications. Fish protein powders have various sanitary processing, purity and functional characteristics which establish them as human food ingredients. Production plants registered for the USA market are located in Peru and France.

Hypoallergenic dog food diets are created for dogs that experience food-related allergies causing adverse effects to their physical health.Super Hypoallergenic is enzymatic hydrolyzed hypoallergenic ostrich protein. The molecules that usually become allergens are intact proteins or glycoproteins. Hypoallergenic dog food diets offer a variety of protein sources that are unique by using proteins that are not recognized by the dog's antibodies as being antigens, minimizing allergic reactions for example Ostrich meat, bones and sinews. Adding novel protein sources, such as novel meats that a dog or its ancestors have never been exposed to is one method. Novel proteins can also be created by chemically modifying well known protein sources using hydrolysis techniques, rendering proteins unrecognizable by the gastrointestinal tract. Not all antigens are specific to proteins, however, and it is possible for anything that the body ingests to become an allergen. Providing diets with a limited amount of ingredients can be used for diagnostic purposes, as well as for dogs who are allergic to the common ingredients that are used in pet food. Certain nutrients are commonly incorporated into hypoallergenic dog food to help alleviate the symptoms of an allergic reaction. These ingredients include omega-3 fatty acids, Vitamins A and E, zinc, novel carbohydrates, and fiber.

Corn sauce or fermented corn sauce is produced by fermentation using corn starch as the primary substrate. It is used as a food condiment and ingredient, both in paste and in powder form. Corn sauce, like soy sauce, has a characteristic savory taste. It is used to flavor dishes including soups, broths, and gravies.














