
Allergies, also known as allergic diseases, are various conditions caused by hypersensitivity of the immune system to typically harmless substances in the environment. These diseases include hay fever, food allergies, atopic dermatitis, allergic asthma, and anaphylaxis. Symptoms may include red eyes, an itchy rash, sneezing, coughing, a runny nose, shortness of breath, or swelling. Note that food intolerances and food poisoning are separate conditions.
An allergen is a type of antigen that produces an abnormally vigorous immune response in which the immune system fights off a perceived threat that would otherwise be harmless to the body. Such reactions are called allergies.
Perfume is a mixture of fragrant essential oils or aroma compounds (fragrances), fixatives and solvents, usually in liquid form, used to give the human body, animals, food, objects, and living-spaces an agreeable scent. Perfumes can be defined as substances that emit and diffuse a pleasant and fragrant odor. They consist of manmade mixtures of aromatic chemicals and essential oils. The 1939 Nobel Laureate for Chemistry, Leopold Ružička stated in 1945 that "right from the earliest days of scientific chemistry up to the present time, perfumes have substantially contributed to the development of organic chemistry as regards methods, systematic classification, and theory."

Allergic rhinitis, of which the seasonal type is called hay fever, is a type of inflammation in the nose that occurs when the immune system overreacts to allergens in the air. Signs and symptoms include a runny or stuffy nose, sneezing, red, itchy, and watery eyes, and swelling around the eyes. The fluid from the nose is usually clear. Symptom onset is often within minutes following allergen exposure, and can affect sleep and the ability to work or study. Some people may develop symptoms only during specific times of the year, often as a result of pollen exposure. Many people with allergic rhinitis also have asthma, allergic conjunctivitis, or atopic dermatitis.

Contact dermatitis is a type of acute or chronic inflammation of the skin caused by exposure to chemical or physical agents. Symptoms of contact dermatitis can include itchy or dry skin, a red rash, bumps, blisters, or swelling. These rashes are not contagious or life-threatening, but can be very uncomfortable.

An aroma compound, also known as an odorant, aroma, fragrance or flavoring, is a chemical compound that has a smell or odor. For an individual chemical or class of chemical compounds to impart a smell or fragrance, it must be sufficiently volatile for transmission via the air to the olfactory system in the upper part of the nose. As examples, various fragrant fruits have diverse aroma compounds, particularly strawberries which are commercially cultivated to have appealing aromas, and contain several hundred aroma compounds.
Cyclohexa-1,3-diene (also known as Benzane) is an organic compound with the formula (C2H4)(CH)4. It is a colorless, flammable liquid. Its refractive index is 1.475 (20 °C, D). It is one of two isomers of cyclohexadiene, the other being 1,4-cyclohexadiene.

Nummular dermatitis is one of the many forms of dermatitis. It is characterized by round or oval-shaped itchy lesions. The name comes from the Latin word "nummus," which means "coin."

Piperonal, also known as heliotropin, is an organic compound which is commonly found in fragrances and flavors. The molecule is structurally related to other aromatic aldehydes such as benzaldehyde and vanillin.

Limonene is a colorless liquid aliphatic hydrocarbon classified as a cyclic monoterpene, and is the major component in the volatile oil of citrus fruit peels. The (+)-isomer, occurring more commonly in nature as the fragrance of oranges, is a flavoring agent in food manufacturing. It is also used in chemical synthesis as a precursor to carvone and as a renewables-based solvent in cleaning products. The less common (-)-isomer has a piny, turpentine-like odor, and is found in the edible parts of such plants as caraway, dill, and bergamot orange plants.

A patch test is a diagnostic method used to determine which specific substances cause allergic inflammation of a patient's skin.

Myrcene, or β-myrcene, is a monoterpene. A colorless oil, it occurs widely in essential oils. It is produced mainly semi-synthetically from Myrcia, from which it gets its name. It is an intermediate in the production of several fragrances. α-Myrcene is the name for the isomer 2-methyl-6-methylene-1,7-octadiene, which has not been found in nature.

Allergic contact dermatitis (ACD) is a form of contact dermatitis that is the manifestation of an allergic response caused by contact with a substance; the other type being irritant contact dermatitis (ICD).

Balsam of Peru or Peru balsam, also known and marketed by many other names, is a balsam derived from a tree known as Myroxylon balsamum var. pereirae; it is found in El Salvador, where it is an endemic species.

p-Toluenesulfonic acid (PTSA, pTSA, or pTsOH) or tosylic acid (TsOH) is an organic compound with the formula CH3C6H4SO3H. It is a white extremely hygroscopic solid that is soluble in water, alcohols, and other polar organic solvents. The CH3C6H4SO2 group is known as the tosyl group and is often abbreviated as Ts or Tos. Most often, TsOH refers to the monohydrate, TsOH.H2O.

Skin allergy testing comprises a range of methods for medical diagnosis of allergies that attempts to provoke a small, controlled, allergic response.

Thiuram disulfides are a class of organosulfur compounds with the formula (R2NCSS)2. Many examples are known, but popular ones include R = Me and R = Et. They are disulfides obtained by oxidation of the dithiocarbamates. These compounds are used in sulfur vulcanization of rubber as well as in the manufacture of pesticides and drugs. They are typically white or pale yellow solids that are soluble in organic solvents.
Allergen of the Year is an annual "award" voted upon by the American Contact Dermatitis Society. The purpose of the award is "to draw attention to the agents causing the most significant clinical effects, those that are underrecognized and those that have become obsolete or for which exposure patterns have changed".
Perfume intolerance or perfume allergy is a condition wherein people exhibit sensitivity or allergic reactions to ingredients in some perfumes and some other fragrances. It is a form of multiple chemical sensitivity, a more general phenomenon for this diagnosis.
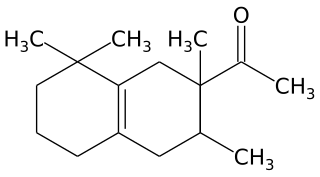
Tetramethyl acetyloctahydronaphthalenes is a synthetic ketone fragrance also known as OTNE and by other commercial trade names such as: Iso E Super, Iso Gamma Super, Anthamber, Amber Fleur, Boisvelone, Iso Ambois, Amberlan, Iso Velvetone, Orbitone, Amberonne. It is a synthetic woody odorant and is used as a fragrance ingredient in perfumes, laundry products and cosmetics.















