Related Research Articles
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.
In organic chemistry, dihydroxybenzenes (benzenediols) are organic compounds in which two hydroxyl groups are substituted onto a benzene ring. These aromatic compounds are classed as phenols. There are three structural isomers: 1,2-dihydroxybenzene is commonly known as catechol, 1,3-dihydroxybenzene is commonly known as resorcinol, and 1,4-dihydroxybenzene is commonly known as hydroquinone.

Biancaea sappan is a species of flowering tree in the legume family, Fabaceae, that is native to tropical Asia. Common names in English include sappanwood and Indian redwood. It was previously ascribed to the genus Caesalpinia. Sappanwood is related to brazilwood, and was itself called brasilwood in the Middle Ages.

1,2-Naphthoquinone or ortho-naphthoquinone is a polycyclic aromatic organic compound with formula C
10H
6O
2. This yellow solid is prepared by oxidation of 1-amino-2-hydroxynaphthalene with ferric chloride.

Juglone, also called 5-hydroxy-1,4-naphthalenedione (IUPAC) is a phenolic organic compound with the molecular formula C10H6O3. In the food industry, juglone is also known as C.I. Natural Brown 7 and C.I. 75500. It is insoluble in benzene but soluble in dioxane, from which it crystallizes as yellow needles. It is an isomer of lawsone, which is the active dye compound in the henna leaf.
Xylenols are organic compounds with the formula (CH3)2C6H3OH. They are volatile colorless solids or oily liquids. They are derivatives of phenol with two methyl groups at various positions relative to the hydroxyl group. Six isomers exist, of which 2,6-xylenol with both methyl groups in an ortho position with respect to the hydroxyl group is the most important. The name xylenol is a portmanteau of the words xylene and phenol.

The Dakin oxidation (or Dakin reaction) is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde (2-hydroxybenzaldehyde or 4-hydroxybenzaldehyde) or ketone reacts with hydrogen peroxide (H2O2) in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidised, whereas the H2O2 is reduced.

Lawsone (2-hydroxy-1,4-naphthoquinone), also known as hennotannic acid, is a red-orange dye present in the leaves of the henna plant, for which it is named, as well as in the common walnut and water hyacinth. Humans have used henna extracts containing lawsone as hair and skin dyes for more than 5,000 years. Lawsone reacts chemically with the protein keratin in skin and hair via a Michael addition reaction, resulting in a strong permanent stain that lasts until the skin or hair is shed. Darker colored staining is due to more lawsone–keratin interactions occurring, which evidently break down as the concentration of lawsone decreases and the tattoo fades. Lawsone strongly absorbs UV light, and aqueous extracts can be effective sunless tanning agents and sunscreens. Lawsone is a 1,4-naphthoquinone derivative, an analog of hydroxyquinone containing one additional ring.
In enzymology, juglone 3-monooxygenase (EC 1.14.99.27) is an enzyme that catalyzes the chemical reaction
1-Naphthol, or α-naphthol, is a organic compound with the formula C10H7OH. It is a fluorescent white solid. 1-Naphthol differs from its isomer 2-naphthol by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol. Both isomers are soluble in simple organic solvents. They are precursors to a variety of useful compounds.
A dihydroxyanthraquinone is any of several isomeric organic compounds with formula C
14H
8O
4, formally derived from 9,10-anthraquinone by replacing two hydrogen atoms by hydroxyl groups. Dihyroxyantraquinones have been studied since the early 1900s, and include some compounds of historical and current importance. The isomers differ in the position of the hydroxyl groups, and of the carbonyl oxygens (=O) of the underlying anthraquinone.
A dihydroxynaphthoquinone is any of several organic compounds that can be viewed as derivatives of naphthoquinone through replacement of two hydrogen atoms (H) by hydroxyl groups (OH).
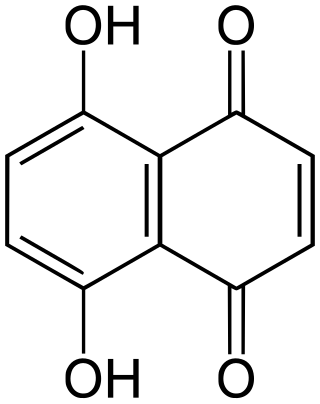
Naphthazarin, often called 5,8-dihydroxy-1,4-naphthoquinone or 5,8-dihydroxy-1,4-naphthalenedione (IUPAC), is a naturally occurring organic compound with formula C
10H
6O
4, formally derived from 1,4-naphthoquinone through replacement of two hydrogen atoms by hydroxyl (OH) groups. It is thus one of many dihydroxynaphthoquinone structural isomers.

Hydroxyquinone often refers to a hydroxybenzoquinone, any organic compound with formula C
6H
4O
3 which can be viewed as a derivative of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH). When unqualified, the terms usually mean specifically the compound 2-hydroxy-1,4-benzoquinone, derived from 1,4-benzoquinone. That parent is sometimes simply called quinone, and this is the only hydroxy derivative of it.
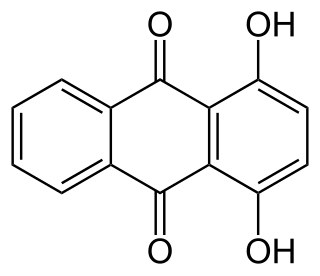
A hydroxyanthraquinone (formula: C14H7O2(OH)) is any of several organic compounds that can be viewed as derivatives of an anthraquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).
A hydroxybenzoquinone is any of several organic compounds that can be viewed as derivatives of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).

Naphthoquinones constitute a class of organic compounds structurally related to naphthalene. Two isomers are common for the parent naphthoquinones:

1,4-Naphthoquinone or para-naphthoquinone is a quinone derived from naphthalene. It forms volatile yellow triclinic crystals and has a sharp odor similar to benzoquinone. It is almost insoluble in cold water, slightly soluble in petroleum ether, and more soluble in polar organic solvents. In alkaline solutions it produces a reddish-brown color. Vitamin K is a derivative of 1,4-naphthoquinone. It is a planar molecule with one aromatic ring fused to a quinone subunit. It is an isomer of 1,2-naphthoquinone.
A cyclohexanetetrol is a chemical compound consisting of a cyclohexane molecule with four hydroxyl groups (–OH) replacing four of the twelve hydrogen atoms. It is therefore a cyclitol. Its generic formula is C
6H
12O
4 or C
6H
8(OH)
4.

1,2,3,4-Cyclohexanetetrol (also named cyclohexane-1,2,3,4-tetrol, 1,2,3,4-tetrahydroxycyclohexane, or ortho-cyclohexanetetrol) is an organic compound whose molecule can be described as a cyclohexane with four hydroxyl (OH) groups substituted for hydrogen atoms on four consecutive carbon atoms. Its formula can be written C
6H
12O
4, C
6H
8(OH)
4, or (–CH(OH)–)4(–CH
2–)2.
References
- ↑ Khalafy, J.; Bruce, J. M. (2002). "Oxidative Dehydrogenation of 1-Tetralones: Synthesis of Juglone, Naphthazarin, and α-Hydroxyanthraquinones" (pdf). Journal of Sciences, Islamic Republic of Iran. 13 (2): 131–139.
- ↑ Thomson, R. H. (1971). Naturally Occurring Quinones . London: Academic Press. Quoted by Khalafy and Bruce.
- ↑ Thomson, R. H. (1987). Naturally Occurring Quinones III. London: Chapman and Hall. Quoted by Khalafy and Bruce.
- ↑ Taylor, R. T.; Flood, L. A. (1983). "Polystyrene-Bound Phenylseleninic Acid: Catalytic Oxidations of Olefins, Ketones, and Aromatic Systems". The Journal of Organic Chemistry. 48 (26): 5160–5164. doi:10.1021/jo00174a003.
- ↑ Lim, M.-Y.; Jeon, J.-H.; Jeong, E. Y.; Lee, C. H.; Lee, H.-S. (2007). "Antimicrobial Activity of 5-Hydroxy-1,4-Naphthoquinone Isolated from Caesalpinia sappan toward Intestinal Bacteria". Food Chemistry. 100 (3): 1254–1258. doi:10.1016/j.foodchem.2005.12.009.
- ↑ Teuber, H.-J.; Götz, N. (1954). "Reaktionen mit Nitrosodisulfonat, V. Mitteilung: Über die Bildung von Naphtochinonen". Chemische Berichte. 87 (9): 1236–1251. doi:10.1002/cber.19540870908.
- ↑ Brahmia, O.; Richard, C. (2005). "Photochemical Transformation of 1-Naphthol in Aerated Aqueous Solution". Photochemical & Photobiological Sciences. 4 (6): 454–458. doi: 10.1039/B504309C . PMID 15920628.
- ↑ Garge, P.; Padhye, S.; Tuchagues, J.-P. (1989). "Iron(II) Complexes of ortho-Functionalized para-Naphthoquinones 1. Synthesis, Characterization, Electronic Structure and Magnetic Properties". Inorganica Chimica Acta. 157 (2): 239–249. doi:10.1016/S0020-1693(00)80548-4.
- ↑ Rane, S. Y.; Ahmed, K.; Salunke-Gawali, S. (2006). "Temperature Effect on Ancillary μ-Carbonato Ligand Modes in Hydroxy Naphthoquinonato Copper(II) Complex: An EPR Spectroscopic and Magnetic Coupling Evidences". Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry. 36 (5): 391–398. doi:10.1080/15533170600729037.