Related Research Articles

The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive elements polonium (Po) and livermorium (Lv). Often, oxygen is treated separately from the other chalcogens, sometimes even excluded from the scope of the term "chalcogen" altogether, due to its very different chemical behavior from sulfur, selenium, tellurium, and polonium. The word "chalcogen" is derived from a combination of the Greek word khalkόs (χαλκός) principally meaning copper, and the Latinized Greek word genēs, meaning born or produced.
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.
Chalcogenide glass is a glass containing one or more chalcogens. Such glasses are covalently bonded materials and may be classified as covalent network solids. Polonium is also a chalcogen but is not used because of its strong radioactivity. Chalcogenide materials behave rather differently from oxides, in particular their lower band gaps contribute to very dissimilar optical and electrical properties.

In chemistry tellurate is a compound containing an oxyanion of tellurium where tellurium has an oxidation number of +6. In the naming of inorganic compounds it is a suffix that indicates a polyatomic anion with a central tellurium atom.

A chalcogenide is a chemical compound consisting of at least one chalcogen anion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfides, selenides, tellurides, and polonides, rather than oxides. Many metal ores exist as chalcogenides. Photoconductive chalcogenide glasses are used in xerography. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide MoS2 is a common solid lubricant.
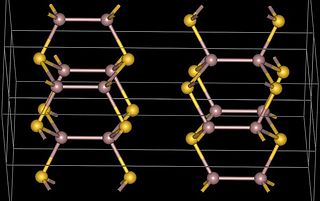
Gallium(II) telluride, GaTe, is a chemical compound of gallium and tellurium. There is research interest in the structure and electronic properties of GaTe because of the possibility that it, or related compounds, may have applications in the electronics industry. Gallium telluride can be made by reacting the elements or by metal organic vapour deposition (MOCVD).

Indium(III) oxide (In2O3) is a chemical compound, an amphoteric oxide of indium.

Tin selenide, also known as stannous selenide, is an inorganic compound with the formula SnSe. Tin(II) selenide is a typical layered metal chalcogenide as it includes a group 16 anion (Se2−) and an electropositive element (Sn2+), and is arranged in a layered structure. Tin(II) selenide is a narrow band-gap (IV-VI) semiconductor structurally analogous to black phosphorus. It has received considerable interest for applications including low-cost photovoltaics, and memory-switching devices.
There are three sets of Indium halides, the trihalides, the monohalides, and several intermediate halides. In the monohalides the oxidation state of indium is +1 and their proper names are indium(I) fluoride, indium(I) chloride, indium(I) bromide and indium(I) iodide.
Indium(III) selenide is a compound of indium and selenium. It has potential for use in photovoltaic devices and it has been the subject of extensive research. The two most common phases, α and β, have a layered structure, while γ is a "defect wurtzite structure." In all, five polymorpsare known: α, β, γ, δ, κ. The α- β phase transition is accompanied by a change in electrical conductivity. The band-gap of γ-In2Se3 is approximately 1.9 eV.

Gallium(II) selenide (GaSe) is a chemical compound. It has a hexagonal layer structure, similar to that of GaS. It is a photoconductor, a second harmonic generation crystal in nonlinear optics, and has been used as a far-infrared conversion material at 14–31 THz and above.

Antimony telluride is an inorganic compound with the chemical formula Sb2Te3. As is true of other pnictogen chalcogenide layered materials, it is a grey crystalline solid with layered structure. Layers consist of two atomic sheets of antimony and three atomic sheets of tellurium and are held together by weak van der Waals forces. Sb2Te3 is a narrow-gap semiconductor with a band gap 0.21 eV; it is also a topological insulator, and thus exhibits thickness-dependent physical properties.
Phosphorus selenides are a relatively obscure group of compounds. There have been some studies of the phosphorus - selenium phase diagram and the glassy amorphous phases are reported. The compounds that have been reported are shown below. While some of phosphorus selenides are similar to their sulfide analogues, there are some new forms, molecular P2Se5 and the polymeric catena-[P4Se4]x. There is also some doubt about the existence of molecular P4Se10.
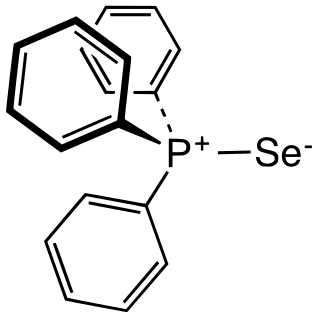
Triphenylphosphine selenide is an organophosphorus compound with the formula (C6H5)3PSe. It is a white solid which is soluble in most organic solvents. The compound is used in the preparation of other selenium compounds and is itself prepared by the reaction of triphenylphosphine with potassium selenocyanate. Single crystals have been isolated with both monoclinic and triclinic structures (space groups: P21/c and P1 respectively); in both cases the geometry at phosphorus is tetrahedral.
Indium(II) selenide (InSe) is an inorganic compound composed of indium and selenium. It is a III-VI layered semiconductor. The solid has a structure consisting of two-dimensional layers bonded together only by van der Waals forces. Each layer has the atoms in the order Se-In-In-Se.
The selenide iodides are chemical compounds that contain both selenide ions (Se2−) and iodide ions (I−) and one or metal atoms. They are in the class of mixed anion compounds or chalcogenide halides.
The telluride iodides are chemical compounds that contain both telluride ions (Te2−) and iodide ions (I−). They are in the class of mixed anion compounds or chalcogenide halides.
A tellurite fluoride is a mixed anion compound containing tellurite and fluoride ions. They have also been called oxyfluorotellurate(IV) where IV is the oxidation state of tellurium in tellurite.
A tellurite tellurate is a chemical compound or salt that contains tellurite and tellurate anions (TeO32- and TeO42-). These are mixed anion compounds. Some have third anions.
Tellurogallates are chemical compounds which contain anionic units of tellurium connected to gallium. They can be considered as gallates where tellurium substitutes for oxygen. Similar compounds include the thiogallates, selenogallates, telluroaluminates, telluroindates and thiostannates. They are in the category of chalcogenotrielates or more broadly tellurometallates or chalcogenometallates.
References
- ↑ Hogg, J. H. C.; Sutherland, H. H.; Williams, D. J. (1973). "The crystal structure of tetraindium triselenide". Acta Crystallographica Section B. 29 (8): 1590. doi:10.1107/S0567740873005108.
- ↑ Schwarz, U.; Hillebrecht, H.; Deiseroth, H. J.; Walther, R. (1995). "In4Te3 und In4Se3: Neubestimmung der Kristallstrukturen, druckabhängiges Verhalten und eine Bemerkung zur Nichtexistenz von In4S3". Zeitschrift für Kristallographie. 210 (5): 342. Bibcode:1995ZK....210..342S. doi:10.1524/zkri.1995.210.5.342.
- ↑ Pfeifer, H.; Deiseroth, H. J. (1991). "In5S4 = SnIn4S4 : Eine Korrektur!". Zeitschrift für Kristallographie - Crystalline Materials. 196 (1–4). doi:10.1524/zkri.1991.196.14.197.
- ↑ Gibson, G. A.; Chaiken, A.; Nauka, K.; Yang, C. C.; Davidson, R.; Holden, A.; Bicknell, R.; Yeh, B. S.; Chen, J.; Liao, H.; Subramanian, S.; Schut, D.; Jasinski, J.; Liliental-Weber, Z. (2005). "Phase-change recording medium that enables ultrahigh-density electron-beam data storage". Applied Physics Letters. 86 (5): 051902. Bibcode:2005ApPhL..86e1902G. doi: 10.1063/1.1856690 . hdl:2144/28192.
- ↑ Zapata-Torres, M. (2001). "Grown of InTe films by close spaced vapor transport". Superficies y Vacío. 13: 69–71.
- ↑ Hogg, J. H. C. (1971). "The crystal structure of In6Se7" (PDF). Acta Crystallographica Section B. 27 (8): 1630–1634. doi:10.1107/S056774087100445X.
- ↑ Hogg, J. H. C.; Duffin, W. J. (1967). "The crystal structure of In6S7". Acta Crystallographica. 23: 111–118. doi:10.1107/S0365110X6700221X.
- ↑ Gamal, G. A. (1997). "On the conduction mechanism and thermoelectric phenomena in In6S7 layer crystals". Crystal Research and Technology. 32 (5): 723–731. doi:10.1002/crat.2170320517.
- ↑ Geller, S.; Hull, G. (1964). "Superconductivity of Intermetallic Compounds with NaCl-Type and Related Structures". Physical Review Letters. 13 (4): 127. Bibcode:1964PhRvL..13..127G. doi:10.1103/PhysRevLett.13.127.
- ↑ Karakostas, T.; Flevaris, N. F.; Vlachavas, N.; Bleris, G. L.; Economou, N. A. (1978). "The ordered state of In3Te4". Acta Crystallographica Section A. 34 (1): 123–126. Bibcode:1978AcCrA..34..123K. doi:10.1107/S0567739478000224.
- ↑ Deiseroth, H. J.; Müller, H. -D. (1995). "Crystal structures of heptagallium decatelluride, Ga7Te10 and heptaindium decatelluride, In7Te10". Zeitschrift für Kristallographie. 210 (1): 57. Bibcode:1995ZK....210...57D. doi:10.1524/zkri.1995.210.1.57.
- ↑ Deiseroth, H. J.; Amann, P.; Thurn, H. (1996). "Die Pentatelluride M2Te5 (M=Al, Ga, In) Polymorphie, Strukturbeziehungen und Homogenitätsbereiche". Zeitschrift für anorganische und allgemeine Chemie. 622 (6): 985. doi:10.1002/zaac.19966220611.