
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles.
Pyrimidine is an aromatic, heterocyclic, organic compound similar to pyridine. One of the three diazines, it has nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine and pyridazine.

Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.
Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2. It is a symmetrical molecule with point group D2h. Pyrazine is less basic than pyridine, pyridazine and pyrimidine. It is a "deliquescent crystal or wax-like solid with a pungent, sweet, corn-like, nutty odour".

Indazole, also called isoindazole, is a heterocyclic aromatic organic compound. This bicyclic compound consists of the fusion of benzene and pyrazole.

Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a pKa of 0.8, compared to 7 for imidazole.

Pyridazine is an aromatic, heterocyclic, organic compound with the molecular formula C4H4N2. It contains a six-membered ring with two adjacent nitrogen atoms. It is a colorless liquid with a boiling point of 208 °C. It is isomeric with two other diazine rings, pyrimidine and pyrazine.
1,3,5-Triazine, also called s-triazine, is an organic chemical compound with the formula (HCN)3. It is a six-membered heterocyclic aromatic ring, one of several isomeric triazines. S-triazine—the "symmetric" isomer—and its derivatives are useful in a variety of applications.
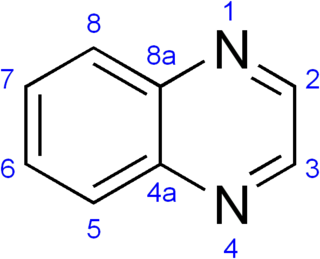
A quinoxaline, also called a benzopyrazine, in organic chemistry, is a heterocyclic compound containing a ring complex made up of a benzene ring and a pyrazine ring. It is isomeric with other naphthyridines including quinazoline, phthalazine and cinnoline. It is a colorless oil that melts just above room temperature. Although quinoxaline itself is mainly of academic interest, quinoxaline derivatives are used as dyes, pharmaceuticals, and antibiotics such as olaquindox, carbadox, echinomycin, levomycin and actinoleutin.
The Friedländer synthesis is a chemical reaction of 2-aminobenzaldehydes with ketones to form quinoline derivatives. It is named after German chemist Paul Friedländer (1857–1923).
Azetidine is a saturated heterocyclic organic compound containing three carbon atoms and one nitrogen atom. It is a liquid at room temperature with a strong odor of ammonia and is strongly basic compared to most secondary amines.
Pyrylium is a cation with formula C5H5O+, consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aromatic character. In particular, because of the positive charge, the oxygen atom is trivalent. Pyrilium is a mono-cyclic and heterocyclic compound, one of the oxonium ions.

Charles Wayne Rees CBE FRS FRSC was a British organic chemist.
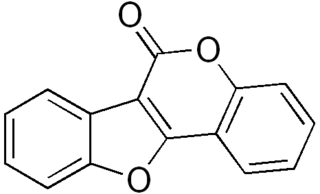
Coumestan is a heterocyclic organic compound. Coumestan forms the central core of a variety of natural compounds known collectively as coumestans. Coumestans are oxidation products of pterocarpan that are similar to coumarin. Coumestans, including coumestrol, a phytoestrogen, are found in a variety of plants. Food sources high in coumestans include split peas, pinto beans, lima beans, and especially alfalfa and clover sprouts.
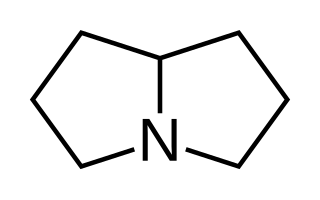
Pyrrolizidine is a heterocyclic organic compound that forms the central chemical structure of a variety of alkaloids known collectively as pyrrolizidine alkaloids. It is one of five classes of iminosugars. These are often synthesized from a carbohydrate.

Indolizidine is a heterocyclic chemical compound that forms the central core of the indolizidine alkaloids such as swainsonine and castanospermine.
The Charles Rees Award is granted by the Royal Society of Chemistry to "reward excellence in the field of heterocyclic chemistry". It was established in 2008 and is awarded biennially. The winner receives £2000, a medal and a certificate, and delivers a lecture at the Lakeland Symposium, Grasmere, UK. Winners are chosen by the Heterocyclic and Synthesis Group, overseen by the Organic Division Awards Committee.
A ring forming reaction or ring-closing reaction in organic chemistry is a general term for a variety of reactions that introduce one or more rings into a molecule. A heterocycle forming reaction is such a reaction that introduces a new heterocycle. Important classes of ring forming reactions include annulations and cycloadditions.
Satinder Vir Kessar is an Indian synthetic organic chemist, academic and an Emeritus professor of Panjab University. He is known for his researches in steroidal and heterocyclic chemistry. He is an elected fellow of The World Academy of Sciences and all the three major Indian science academies, viz. The Indian National Science Academy, the Indian Academy of Sciences and the National Academy of Sciences, India. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 1972, for his contributions to chemical sciences.
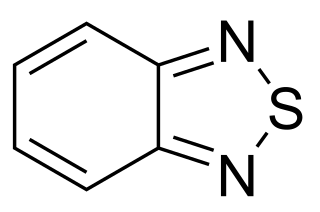
2,1,3-Benzothiadiazole is a bicyclic molecule composed of a benzene ring that is fused to a 1,2,5-thiadiazole.












