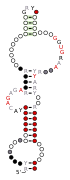
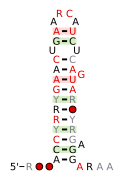- A consensus secondary structure and primary sequence for the C-loop RNA motif.
- A consensus secondary structure and primary sequence for the docking elbow RNA motif.
- A consensus secondary structure and primary sequence for the kink-turn RNA motif.
- A consensus secondary structure and primary sequence for the kink-turn RNA motif.
- A consensus secondary structure and primary sequence for the right-angle RNA motif.
- A consensus secondary structure and primary sequence for the 3` Sarcin-Ricin (or bulged-G) RNA motif. [6]
- A consensus secondary structure and primary sequence for the 5` Sarcin-Ricin (or bulged-G) RNA motif. [6]
- A consensus secondary structure and primary sequence for the twist-up RNA motif. [9]
- A consensus secondary structure and primary sequence for the UAA-GAN RNA motif. [10]
Related Research Articles

In biology, the SECIS element is an RNA element around 60 nucleotides in length that adopts a stem-loop structure. This structural motif directs the cell to translate UGA codons as selenocysteines. SECIS elements are thus a fundamental aspect of messenger RNAs encoding selenoproteins, proteins that include one or more selenocysteine residues.

Biomolecular structure is the intricate folded, three-dimensional shape that is formed by a molecule of protein, DNA, or RNA, and that is important to its function. The structure of these molecules may be considered at any of several length scales ranging from the level of individual atoms to the relationships among entire protein subunits. This useful distinction among scales is often expressed as a decomposition of molecular structure into four levels: primary, secondary, tertiary, and quaternary. The scaffold for this multiscale organization of the molecule arises at the secondary level, where the fundamental structural elements are the molecule's various hydrogen bonds. This leads to several recognizable domains of protein structure and nucleic acid structure, including such secondary-structure features as alpha helixes and beta sheets for proteins, and hairpin loops, bulges, and internal loops for nucleic acids. The terms primary, secondary, tertiary, and quaternary structure were introduced by Kaj Ulrik Linderstrøm-Lang in his 1951 Lane Medical Lectures at Stanford University.
Nucleic acid structure prediction is a computational method to determine secondary and tertiary nucleic acid structure from its sequence. Secondary structure can be predicted from one or several nucleic acid sequences. Tertiary structure can be predicted from the sequence, or by comparative modeling.
A release factor is a protein that allows for the termination of translation by recognizing the termination codon or stop codon in an mRNA sequence. They are named so because they release new peptides from the ribosome.

In molecular biology, SNORD15 is a non-coding RNA (ncRNA) molecule which functions in the modification of small nuclear RNAs. This type of modifying RNA is usually located in the nucleolus of the eukaryotic cell which is a major site of snRNA biogenesis. It is known as a small nucleolar RNA (snoRNA) and also often referred to as a guide RNA.
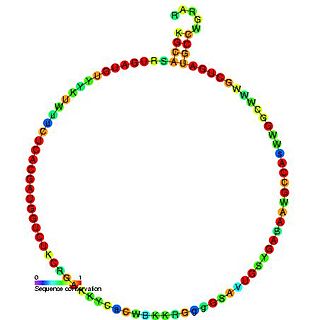
In molecular biology, snoRNA U35 is a non-coding RNA (ncRNA) molecule which functions in the modification of other small nuclear RNAs (snRNAs). This type of modifying RNA is usually located in the nucleolus of the eukaryotic cell which is a major site of snRNA biogenesis. It is known as a small nucleolar RNA (snoRNA) and also often referred to as a guide RNA.
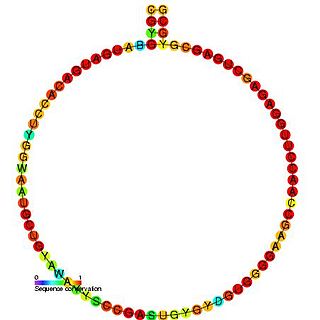
In molecular biology, snoRNA U39 is a non-coding RNA (ncRNA) molecule which functions in the modification of other small nuclear RNAs (snRNAs). This type of modifying RNA is usually located in the nucleolus of the eukaryotic cell which is a major site of snRNA biogenesis. It is known as a small nucleolar RNA (snoRNA) and also often referred to as a guide RNA.
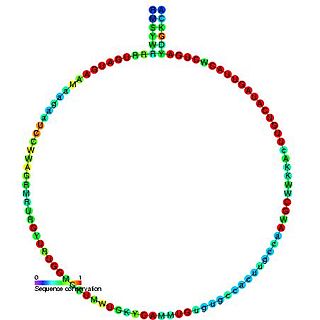
In molecular biology, snoRNA U46 is a non-coding RNA (ncRNA) molecule which functions in the modification of other small nuclear RNAs (snRNAs). This type of modifying RNA is usually located in the nucleolus of the eukaryotic cell which is a major site of snRNA biogenesis. It is known as a small nucleolar RNA (snoRNA) and also often referred to as a guide RNA.
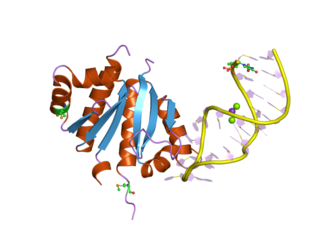
The K Homology (KH) domain is a protein domain that was first identified in the human heterogeneous nuclear ribonucleoprotein (hnRNP) K. An evolutionarily conserved sequence of around 70 amino acids, the KH domain is present in a wide variety of nucleic acid-binding proteins. The KH domain binds RNA, and can function in RNA recognition. It is found in multiple copies in several proteins, where they can function cooperatively or independently. For example, in the AU-rich element RNA-binding protein KSRP, which has 4 KH domains, KH domains 3 and 4 behave as independent binding modules to interact with different regions of the AU-rich RNA targets. The solution structure of the first KH domain of FMR1 and of the C-terminal KH domain of hnRNP K determined by nuclear magnetic resonance (NMR) revealed a beta-alpha-alpha-beta-beta-alpha structure. Autoantibodies to NOVA1, a KH domain protein, cause paraneoplastic opsoclonus ataxia. The KH domain is found at the N-terminus of the ribosomal protein S3. This domain is unusual in that it has a different fold compared to the normal KH domain.
Ribosomal frameshifting, also known as translational frameshifting or translational recoding, is a biological phenomenon that occurs during translation that results in the production of multiple, unique proteins from a single mRNA. The process can be programmed by the nucleotide sequence of the mRNA and is sometimes affected by the secondary, 3-dimensional mRNA structure. It has been described mainly in viruses, retrotransposons and bacterial insertion elements, and also in some cellular genes.

Tetraloops are a type of four-base hairpin loop motifs in RNA secondary structure that cap many double helices. There are many variants of the tetraloop. The published ones include ANYA, CUYG, GNRA, UNAC and UNCG.

EF-G is a prokaryotic elongation factor involved in protein translation. As a GTPase, EF-G catalyzes the movement (translocation) of transfer RNA (tRNA) and messenger RNA (mRNA) through the ribosome.

Nucleic acid structure refers to the structure of nucleic acids such as DNA and RNA. Chemically speaking, DNA and RNA are very similar. Nucleic acid structure is often divided into four different levels: primary, secondary, tertiary, and quaternary.

Nucleic acid secondary structure is the basepairing interactions within a single nucleic acid polymer or between two polymers. It can be represented as a list of bases which are paired in a nucleic acid molecule. The secondary structures of biological DNAs and RNAs tend to be different: biological DNA mostly exists as fully base paired double helices, while biological RNA is single stranded and often forms complex and intricate base-pairing interactions due to its increased ability to form hydrogen bonds stemming from the extra hydroxyl group in the ribose sugar.
16S rRNA (guanine1405-N7)-methyltransferase (EC 2.1.1.179, methyltransferase Sgm, m7G1405 Mtase, Sgm Mtase, Sgm, sisomicin-gentamicin methyltransferase, sisomicin-gentamicin methylase, GrmA, RmtB, RmtC, ArmA) is an enzyme with systematic name S-adenosyl-L-methionine:16S rRNA (guanine1405-N7)-methyltransferase. This enzyme catalyses the following chemical reaction
Éric Westhof is a French biochemist born in Uccle (Belgium) on July 25, 1948. He is a member of the Academie des sciences, head of Education and Training (DEF) and a member of the Board of Directors of the "La Main à la pâte" Foundation. He is professor emeritus of structural biochemistry at the University of Strasbourg at the Institute of Molecular and Cellular Biology.
Non-canonical base pairs are planar hydrogen bonded pairs of nucleobases, having hydrogen bonding patterns which differ from the patterns observed in Watson-Crick base pairs, as in the classic double helical DNA. The structures of polynucleotide strands of both DNA and RNA molecules can be understood in terms of sugar-phosphate backbones consisting of phosphodiester-linked D 2’ deoxyribofuranose sugar moieties, with purine or pyrimidine nucleobases covalently linked to them. Here, the N9 atoms of the purines, guanine and adenine, and the N1 atoms of the pyrimidines, cytosine and thymine, respectively, form glycosidic linkages with the C1’ atom of the sugars. These nucleobases can be schematically represented as triangles with one of their vertices linked to the sugar, and the three sides accounting for three edges through which they can form hydrogen bonds with other moieties, including with other nucleobases. The side opposite to the sugar linked vertex is traditionally called the Watson-Crick edge, since they are involved in forming the Watson-Crick base pairs which constitute building blocks of double helical DNA. The two sides adjacent to the sugar-linked vertex are referred to, respectively, as the Sugar and Hoogsteen edges.
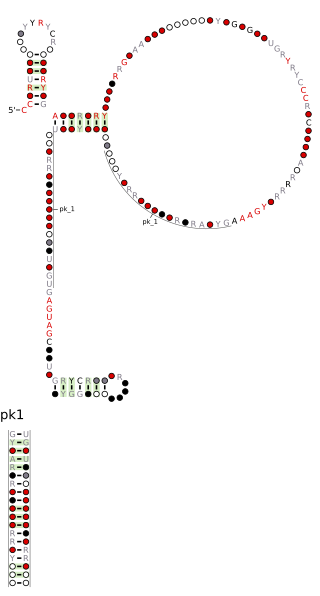
The Pseudomonadales-1 RNA motif is a conserved RNA structure that was discovered by bioinformatics. The Pseudomonadales-1 motif often exhibits an apparent sarcin-ricin loop, a type internal loop common in RNA. Pseudomonadales-1 motif RNAs are found in relatively closely related species of Pseudomonadales. Despite this narrow distribution, the Pseudomonadales-1 RNA motif does not exhibit many invariant nucleotide positions, suggesting that it does not need to be highly conserved at the primary sequence level.
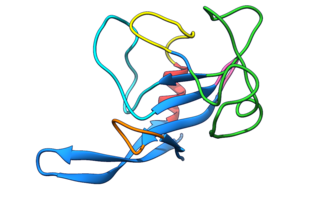
Fungal ribotoxins are a group of extracellular ribonucleases (RNases) secreted by fungi. Their most notable characteristic is their extraordinary specificity. They inactivate ribosomes by cutting a single phosphodiester bond of the rRNA that is found in a universally conserved sequence. This cleavage leads to cell death by apoptosis. However, since they are extracellular proteins, they must first enter the cells that constitute their target to exert their cytotoxic action. This entry constitutes the rate-determining step of their action.
References
- ↑ Lescoute, A; Leontis, NB; Massire, C; Westhof, E (2005). "Recurrent structural RNA motifs, Isostericity Matrices and sequence alignments". Nucleic Acids Research. 33 (8): 2395–409. doi:10.1093/nar/gki535. PMC 1087784 . PMID 15860776.
- ↑ Lehmann, J; Jossinet, F; Gautheret, D (May 1, 2013). "A universal RNA structural motif docking the elbow of tRNA in the ribosome, RNAse P and T-box leaders". Nucleic Acids Research. 41 (10): 5494–502. doi:10.1093/nar/gkt219. PMC 3664808 . PMID 23580544.
- ↑ Klein, D.J. (2001). "The kink-turn: a new RNA secondary structure motif". The EMBO Journal. 20 (15): 4214–4221. doi:10.1093/emboj/20.15.4214. ISSN 1460-2075. PMC 149158 . PMID 11483524.
- ↑ Schroeder, KT; McPhee, SA; Ouellet, J; Lilley, DM (Aug 2010). "A structural database for k-turn motifs in RNA". RNA. 16 (8): 1463–8. doi:10.1261/rna.2207910. PMC 2905746 . PMID 20562215.
- ↑ Grabow, WW; Zhuang, Z; Swank, ZN; Shea, JE; Jaeger, L (Nov 23, 2012). "The right angle (RA) motif: a prevalent ribosomal RNA structural pattern found in group I introns". Journal of Molecular Biology. 424 (1–2): 54–67. doi:10.1016/j.jmb.2012.09.012. PMC 3488136 . PMID 22999957.
- 1 2 3 Szewczak, AA; Moore, PB (Mar 17, 1995). "The sarcin/ricin loop, a modular RNA". Journal of Molecular Biology. 247 (1): 81–98. doi: 10.1006/jmbi.1994.0124 . PMID 7897662.
- ↑ Leontis, NB; Westhof, E (Oct 30, 1998). "A common motif organizes the structure of multi-helix loops in 16 S and 23 S ribosomal RNAs". Journal of Molecular Biology. 283 (3): 571–83. doi:10.1006/jmbi.1998.2106. PMID 9784367.
- ↑ Moore PB (1999). "Structural motifs in RNA". Annu. Rev. Biochem. 68: 287–300. doi:10.1146/annurev.biochem.68.1.287. PMID 10872451.
- 1 2 Zhong, C; Zhang, S (Feb 2012). "Clustering RNA structural motifs in ribosomal RNAs using secondary structural alignment". Nucleic Acids Research. 40 (3): 1307–17. doi:10.1093/nar/gkr804. PMC 3273805 . PMID 21976732.
- 1 2 Lee, JC; Gutell, RR; Russell, R (Jul 28, 2006). "The UAA/GAN internal loop motif: a new RNA structural element that forms a cross-strand AAA stack and long-range tertiary interactions". Journal of Molecular Biology. 360 (5): 978–88. doi:10.1016/j.jmb.2006.05.066. PMID 16828489.