Related Research Articles

The Scandinavian Simvastatin Survival Study, was a multicentre, randomized, double-blind, placebo-controlled clinical trial, which provided the initial data that supported the use of the cholesterol-lowering drug, simvastatin, in people with a moderately raised cholesterol and coronary heart disease (CHD); that is people who had previously had a heart attack or angina. The study was sponsored by the pharmaceutical company Merck and enrolled 4,444 people from 94 centres in Scandinavia.
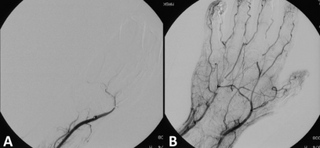
Thrombolysis, also called fibrinolytic therapy, is the breakdown (lysis) of blood clots formed in blood vessels, using medication. It is used in ST elevation myocardial infarction, stroke, and in cases of severe venous thromboembolism.

Alteplase (t-PA) is a thrombolytic medication, used to treat acute ischemic stroke, acute ST-elevation myocardial infarction, pulmonary embolism associated with low blood pressure, and blocked central venous catheter. It is given by injection into a vein or artery. Alteplase is the same as the normal human plasminogen activator produced in vascular endothelial cells and is synthesized via recombinant DNA technology in Chinese hamster ovary cells (CHO). Alteplase causes the breakdown of a clot by inducing fibrinolysis.
Sir Richard Peto is an English statistician and epidemiologist who is Professor of Medical Statistics and Epidemiology at the University of Oxford, England.

Desmoteplase is a novel, highly fibrin-specific "clot-busting" (thrombolytic) drug in development that reached phase III clinical trials. The Danish pharmaceutical company, Lundbeck, owns the worldwide rights to Desmoteplase. In 2009, two large trials were started to test it as a safe and effective treatment for patients with acute ischaemic stroke. After disappointing results in DIAS-3, DIAS-4 was terminated, and in December 2014 Lundbeck announced that they would stop the development of desmoteplase.

A cerebral infarction is the pathologic process that results in an area of necrotic tissue in the brain. It is caused by disrupted blood supply (ischemia) and restricted oxygen supply (hypoxia), most commonly due to thromboembolism, and manifests clinically as ischemic stroke. In response to ischemia, the brain degenerates by the process of liquefactive necrosis.

Percutaneous coronary intervention (PCI) is a non-surgical procedure used to treat narrowing of the coronary arteries of the heart found in coronary artery disease. The process involves combining coronary angioplasty with stenting, which is the insertion of a permanent wire-meshed tube that is either drug eluting (DES) or composed of bare metal (BMS). The stent delivery balloon from the angioplasty catheter is inflated with media to force contact between the struts of the stent and the vessel wall, thus widening the blood vessel diameter. After accessing the blood stream through the femoral or radial artery, the procedure uses coronary catheterization to visualise the blood vessels on X-ray imaging. After this, an interventional cardiologist can perform a coronary angioplasty, using a balloon catheter in which a deflated balloon is advanced into the obstructed artery and inflated to relieve the narrowing; certain devices such as stents can be deployed to keep the blood vessel open. Various other procedures can also be performed.
Anistreplase is a thrombolytic drug. It is also known as anisoylated plasminogen streptokinase activator complex (APSAC). As a thrombolytic drug, it is used to treat blood clots in emergency situations.

Bivalirudin (Bivalitroban), sold under the brand names Angiomax and Angiox and manufactured by The Medicines Company, is a direct thrombin inhibitor (DTI).
Tenecteplase, sold under the trade names TNKase, Metalyse and Elaxim, is an enzyme used as a thrombolytic drug.

Désiré, Baron Collen is a Belgian physician, chemist, biotechnology entrepreneur and life science investor. He made several discoveries in thrombosis, haemostasis and vascular biology in many of which serendipity played a significant role. His main achievement has been his role in the development of tissue-type plasminogen activator (t-PA) from a laboratory concept to a life-saving drug for dissolving blood clots causing acute myocardial infarction or acute ischemic stroke. Recombinant t-PA was produced and marketed by Genentech Inc as Activase and by Boehringer Ingelheim GmbH as Actilyse, and is considered biotechnology's first life saving drug.
The Thrombolysis In Myocardial Infarction, or TIMI Study Group, is an Academic Research Organization (ARO) affiliated with Brigham and Women's Hospital and Harvard Medical School dedicated to advancing the knowledge and care of patients suffering from cardiovascular disease. The TIMI Study Group provides robust expertise in the key aspects of a clinical trial, including academic leadership, global trial management, biostatistics, clinical event adjudication, safety desk, medical hotline, and core laboratories. The group has its headquarters in Boston, Massachusetts.
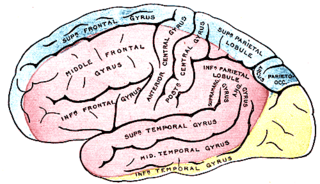
Anterior cerebral artery syndrome is a condition whereby the blood supply from the anterior cerebral artery (ACA) is restricted, leading to a reduction of the function of the portions of the brain supplied by that vessel: the medial aspects of the frontal and parietal lobes, basal ganglia, anterior fornix and anterior corpus callosum.
The Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI) is a cardiology research group founded as a collaboration between two Italian organisations – the Mario Negri Institute and the Associazione Nazionale dei Medici Cardiologi Ospedalieri (ANMCO).

A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops to the coronary artery of the heart, causing damage to the heart muscle. The most common symptom is chest pain or discomfort which may travel into the shoulder, arm, back, neck or jaw. Often it occurs in the center or left side of the chest and lasts for more than a few minutes. The discomfort may occasionally feel like heartburn. Other symptoms may include shortness of breath, nausea, feeling faint, a cold sweat or feeling tired. About 30% of people have atypical symptoms. Women more often present without chest pain and instead have neck pain, arm pain or feel tired. Among those over 75 years old, about 5% have had an MI with little or no history of symptoms. An MI may cause heart failure, an irregular heartbeat, cardiogenic shock or cardiac arrest.
Polycap is a specific five-in-one fixed dose combination polypill created by Cadila Pharmaceuticals Limited of Ahmedabad, India that combines moderate levels of five different medications in a single, one-a-day pill aimed at reducing/preventing heart attacks and strokes.

Reperfusion therapy is a medical treatment to restore blood flow, either through or around, blocked arteries, typically after a heart attack. Reperfusion therapy includes drugs and surgery. The drugs are thrombolytics and fibrinolytics used in a process called thrombolysis. Surgeries performed may be minimally-invasive endovascular procedures such as a percutaneous coronary intervention (PCI), which involves coronary angioplasty. The angioplasty uses the insertion of a balloon to open up the artery, with the possible additional use of one or more stents. Other surgeries performed are the more invasive bypass surgeries that graft arteries around blockages.

Management of acute coronary syndrome is targeted against the effects of reduced blood flow to the afflicted area of the heart muscle, usually because of a blood clot in one of the coronary arteries, the vessels that supply oxygenated blood to the myocardium. This is achieved with urgent hospitalization and medical therapy, including drugs that relieve chest pain and reduce the size of the infarct, and drugs that inhibit clot formation; for a subset of patients invasive measures are also employed. Basic principles of management are the same for all types of acute coronary syndrome. However, some important aspects of treatment depend on the presence or absence of elevation of the ST segment on the electrocardiogram, which classifies cases upon presentation to either ST segment elevation myocardial infarction (STEMI) or non-ST elevation acute coronary syndrome (NST-ACS); the latter includes unstable angina and non-ST elevation myocardial infarction (NSTEMI). Treatment is generally more aggressive for STEMI patients, and reperfusion therapy is more often reserved for them. Long-term therapy is necessary for prevention of recurrent events and complications.
Remote ischemic conditioning (RIC) is an experimental medical procedure that aims to reduce the severity of ischaemic injury to an organ such as the heart or the brain, most commonly in the situation of a heart attack or a stroke, or during procedures such as heart surgery when the heart may temporary suffer ischaemia during the operation, by triggering the body's natural protection against tissue injury. Although noted to have some benefits in experimental models in animals, this is still an experimental procedure in humans and initial evidence from small studies have not been replicated in larger clinical trials. Successive clinical trials have failed to identify evidence supporting a protective role in humans.
A migrainous infarction is a rare type of ischaemic stroke which occurs in correspondence with migraine aura symptoms. Symptoms include headaches, visual disturbances, strange sensations and dysphasia, all of which gradually worsen causing neurological changes which ultimately increase the risk of an ischaemic stroke. Typically, women under the age of 45 who experience migraine with aura (MA) are at the greatest risk for developing migrainous infarction, especially when combined with smoking and use of oral contraceptives.
References
- ↑ Hennekens, Charles H. (1998), "Trials of Thrombolytic Therapy: the International Studies of Infarct Survival Experience", Journal of Interventional Cardiology, 11: 1–7, doi:10.1111/j.1540-8183.1998.tb00089.x
- ↑ Hennekens, Charles H.; Skerrett, P.J. (2005). "International Studies of Infarct Survival (ISIS)". Encyclopedia of Biostatistics. doi:10.1002/0470011815.b2a01031. ISBN 047084907X.
- ↑ ISIS-1 (Second International Study of lnfarct Survival) Collaborative Group (1986), "Randomised trial of intravenous atenolol among 16027 cases of suspected acute myocardial infarction: ISIS-1", The Lancet, 328 (8498): 57–66, doi:10.1016/S0140-6736(86)91607-7
- ↑ ISIS-2 (Second International Study of lnfarct Survival) Collaborative Group (1988), "Randomised Trial of Intravenous Streptokinase, Oral Aspirin, Both, or Neither Among 17 187 Cases of Suspected Acute Myocardial Infarction: ISIS-2", The Lancet, 332 (8607): 349–360, doi:10.1016/S0140-6736(88)92833-4, PMID 2899772
- ↑ ISIS-3 (Third International Study of lnfarct Survival) Collaborative Group (1992), "ISIS-3: a randomised comparison of streptokinase vs tissue plasminogen activator vs anistreplase and of aspirin plus heparin vs aspirin alone among 41 299 cases of suspected acute myocardial infarction", The Lancet, 339 (8796): 753–770, doi:10.1016/0140-6736(92)91893-D
- ↑ ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group (1995), "ISIS-4: A randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58 050 patients with suspected acute myocardial infarction", The Lancet, 345 (8951): 669–682, doi:10.1016/S0140-6736(95)90865-X