
Vanillin is an organic compound with the molecular formula C8H8O3. It is a phenolic aldehyde. Its functional groups include aldehyde, hydroxyl, and ether. It is the primary component of the extract of the vanilla bean. Synthetic vanillin is now used more often than natural vanilla extract as a flavoring in foods, beverages, and pharmaceuticals.

Lobelia is a genus of flowering plants comprising 415 species, with a subcosmopolitan distribution primarily in tropical to warm temperate regions of the world, a few species extending into cooler temperate regions. They are known generally as lobelias.
A glucoside is a glycoside that is derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is hydrolysed by purely chemical means, or decomposed by fermentation or enzymes.

Alpinia nutans, the shellflower, or dwarf cardamom, is a Southeast Asian plant of the ginger family (Zingiberaceae), and is a medicinal plant used to control hypertension, as diuretic, antifungal, and antiulcer. In Japan it is used as food preservative.

Sodium ferulate, the sodium salt of ferulic acid, is a compound used in traditional Chinese medicine thought to be useful for treatment of cardiovascular and cerebrovascular diseases and to prevent thrombosis, although there is no high-quality clinical evidence for such effects. It is found in the root of Angelica sinensis. As of 2005, it was under preliminary clinical research in China. Ferulic acid can also be extracted from the root of the Chinese herb Ligusticum chuanxiong.

Caffeic acid is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups. It is found in all plants because it is an intermediate in the biosynthesis of lignin, one of the principal components of woody plant biomass and its residues.

Ferulic acid is a hydroxycinnamic acid, an organic compound with the formula (CH3O)HOC6H3CH=CHCO2H. The name is derived from the genus Ferula, referring to the giant fennel (Ferula communis). Classified as a phenolic phytochemical, ferulic acid is an amber colored solid. Esters of ferulic acid are found in plant cell walls, covalently bonded to hemicellulose such as arabinoxylans.

p-Coumaric acid is an organic compound with the formula HOC6H4CH=CHCO2H. It is one of the three isomers of hydroxycinnamic acid. It is a white solid that is only slightly soluble in water but very soluble in ethanol and diethyl ether.
Hydroxycinnamic acids (hydroxycinnamates) are a class of aromatic acids or phenylpropanoids having a C6–C3 skeleton. These compounds are hydroxy derivatives of cinnamic acid.

The phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acids phenylalanine and tyrosine. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of coumaric acid, which is the central intermediate in phenylpropanoid biosynthesis. From 4-coumaroyl-CoA emanates the biosynthesis of myriad natural products including lignols, flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. The coumaroyl component is produced from cinnamic acid.
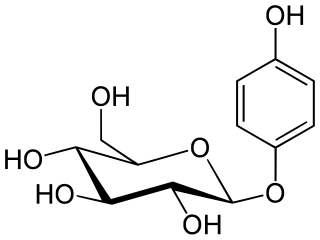
Arbutin is a glycoside; a glycosylated hydroquinone extracted from the bearberry plant in the genus Arctostaphylos among many other medicinal plants, primarily in the family Ericaceae. Applied topically, it inhibits tyrosinase and thus prevents the formation of melanin. Arbutin is therefore used as a skin-lightening agent. Very tiny amounts of arbutin are found in wheat, pear skins, and some other foods. It is also found in Viburnum opulus and Bergenia crassifolia. Arbutin was also produced by an in vitro culture of Schisandra chinensis.

Lobeline is a pyridine alkaloid found in a variety of plants, particularly those in the genus Lobelia, including Indian tobacco, Devil's tobacco, great lobelia, Lobelia chinensis, and Hippobroma longiflora. In its pure form, it is a white amorphous powder which is freely soluble in water.

Diferulic acids (also known as dehydrodiferulic acids) are organic compounds that have the general chemical formula C20H18O8, they are formed by dimerisation of ferulic acid. Curcumin and curcuminoids, though having a structure resembling diferulic acids', are not formed that way but through a condensation process. Just as ferulic acid is not the proper IUPAC name, the diferulic acids also tend to have trivial names that are more commonly used than the correct IUPAC name. Diferulic acids are found in plant cell walls, particularly those of grasses.

Vanillic acid is a dihydroxybenzoic acid derivative used as a flavoring agent. It is an oxidized form of vanillin. It is also an intermediate in the production of vanillin from ferulic acid.

Lobelia chinensis, commonly known as Asian lobelia, Chinese lobelia, and Herba Lobellae Chinensis, is a species of flowering plant in the family Campanulaceae. It is one of the 50 fundamental herbs used in traditional Chinese medicine, where it has the name.

2-Methoxy-4-vinylphenol is an aromatic substance used as a flavoring agent. It is one of the compounds responsible for the natural aroma of buckwheat.

Ethyl propionate is an organic compound with formula C2H5O2CCH2CH3. It is the ethyl ester of propionic acid. It is a colorless volatile liquid with a pineapple-like odor. Some fruits such as kiwis and strawberries contain ethyl propionate in small amounts.

Phenolic acids or phenolcarboxylic acids are types of aromatic acid compounds. Included in that class are substances containing a phenolic ring and an organic carboxylic acid function. Two important naturally occurring types of phenolic acids are hydroxybenzoic acids and hydroxycinnamic acids, which are derived from non-phenolic molecules of benzoic and cinnamic acid, respectively.

Fertaric acid is a hydroxycinnamic acid found in wine and grapes. It is an ester formed from ferulic acid bound to tartaric acid.
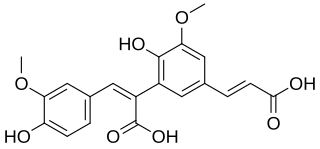
8,5′-Diferulic acid is a non cyclic type of diferulic acid. It is the predominant diferulic acid in sugar beet pulp. It is also found in barley, in maize bran and rye. 8,5′-Diferulic acid has also been identified to be covalently linked to carbohydrate moieties of the arabinogalactan-protein fraction of gum arabic.


















