Related Research Articles

Antidepressants are a class of medications used to treat major depressive disorder, anxiety disorders, chronic pain, and addiction.
Evidence-based medicine (EBM) is "the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients." The aim of EBM is to integrate the experience of the clinician, the values of the patient, and the best available scientific information to guide decision-making about clinical management. The term was originally used to describe an approach to teaching the practice of medicine and improving decisions by individual physicians about individual patients.
Meta-analysis is the statistical combination of the results of multiple studies addressing a similar research question. An important part of this method involves computing an effect size across all of the studies; this involves extracting effect sizes and variance measures from various studies. Meta-analyses are integral in supporting research grant proposals, shaping treatment guidelines, and influencing health policies. They are also pivotal in summarizing existing research to guide future studies, thereby cementing their role as a fundamental methodology in metascience. Meta-analyses are often, but not always, important components of a systematic review procedure. For instance, a meta-analysis may be conducted on several clinical trials of a medical treatment, in an effort to obtain a better understanding of how well the treatment works.

A placebo can be roughly defined as a sham medical treatment. Common placebos include inert tablets, inert injections, sham surgery, and other procedures.

A randomized controlled trial is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures, diets or other medical treatments.
In a blind or blinded experiment, information which may influence the participants of the experiment is withheld until after the experiment is complete. Good blinding can reduce or eliminate experimental biases that arise from a participants' expectations, observer's effect on the participants, observer bias, confirmation bias, and other sources. A blind can be imposed on any participant of an experiment, including subjects, researchers, technicians, data analysts, and evaluators. In some cases, while blinding would be useful, it is impossible or unethical. For example, it is not possible to blind a patient to their treatment in a physical therapy intervention. A good clinical protocol ensures that blinding is as effective as possible within ethical and practical constraints.

A scientific control is an experiment or observation designed to minimize the effects of variables other than the independent variable. This increases the reliability of the results, often through a comparison between control measurements and the other measurements. Scientific controls are a part of the scientific method.
Clinical study design is the formulation of trials and experiments, as well as observational studies in medical, clinical and other types of research involving human beings. The goal of a clinical study is to assess the safety, efficacy, and / or the mechanism of action of an investigational medicinal product (IMP) or procedure, or new drug or device that is in development, but potentially not yet approved by a health authority. It can also be to investigate a drug, device or procedure that has already been approved but is still in need of further investigation, typically with respect to long-term effects or cost-effectiveness.
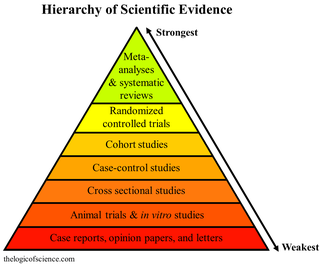
A hierarchy of evidence, comprising levels of evidence (LOEs), that is, evidence levels (ELs), is a heuristic used to rank the relative strength of results obtained from experimental research, especially medical research. There is broad agreement on the relative strength of large-scale, epidemiological studies. More than 80 different hierarchies have been proposed for assessing medical evidence. The design of the study and the endpoints measured affect the strength of the evidence. In clinical research, the best evidence for treatment efficacy is mainly from meta-analyses of randomized controlled trials (RCTs). Systematic reviews of completed, high-quality randomized controlled trials – such as those published by the Cochrane Collaboration – rank the same as systematic review of completed high-quality observational studies in regard to the study of side effects. Evidence hierarchies are often applied in evidence-based practices and are integral to evidence-based medicine (EBM).
In a randomized experiment, allocation concealment hides the sorting of trial participants into treatment groups so that this knowledge cannot be exploited. Adequate allocation concealment serves to prevent study participants from influencing treatment allocations for subjects. Studies with poor allocation concealment are prone to selection bias.

In science, randomized experiments are the experiments that allow the greatest reliability and validity of statistical estimates of treatment effects. Randomization-based inference is especially important in experimental design and in survey sampling.

In fields such as epidemiology, social sciences, psychology and statistics, an observational study draws inferences from a sample to a population where the independent variable is not under the control of the researcher because of ethical concerns or logistical constraints. One common observational study is about the possible effect of a treatment on subjects, where the assignment of subjects into a treated group versus a control group is outside the control of the investigator. This is in contrast with experiments, such as randomized controlled trials, where each subject is randomly assigned to a treated group or a control group. Observational studies, for lacking an assignment mechanism, naturally present difficulties for inferential analysis.

Esketamine, sold under the brand names Spravato and Ketanest among others, is the S(+) enantiomer of ketamine. It is a dissociative hallucinogen drug used as a general anesthetic and as an antidepressant for treatment of depression. Esketamine is the active enantiomer of ketamine in terms of NMDA receptor antagonism and is more potent than racemic ketamine.
A glossary of terms used in clinical research.

Placebo-controlled studies are a way of testing a medical therapy in which, in addition to a group of subjects that receives the treatment to be evaluated, a separate control group receives a sham "placebo" treatment which is specifically designed to have no real effect. Placebos are most commonly used in blinded trials, where subjects do not know whether they are receiving real or placebo treatment. Often, there is also a further "natural history" group that does not receive any treatment at all.

Dupilumab, sold under the brand name Dupixent, is a monoclonal antibody blocking interleukin 4 and interleukin 13, used for allergic diseases such as atopic dermatitis (eczema), asthma and nasal polyps which result in chronic sinusitis. It is also used for the treatment of eosinophilic esophagitis and prurigo nodularis.
A significant amount of research has been performed on glycosaminoglycans, especially glucosamine and chondroitin, for the treatment of arthritis. These compounds are commonly marketed as nutritional supplements and numerous 'soft therapeutic claims' are made about their health benefits - especially in aging populations. Since glucosamine is a precursor for glycosaminoglycans, and glycosaminoglycans are major components of cartilage, ingesting glucosamine might nourish joints, and thereby alleviate arthritis symptoms. Authoritative opinions on the actual therapeutic value of these compounds have been very mixed.
Isabelle Boutron is a professor of epidemiology at the Université Paris Cité and head of the INSERM- METHODS team within the Centre of Research in Epidemiology and Statistics (CRESS). She was originally trained in rheumatology and later switched to a career in epidemiology and public health. She is also deputy director of the French EQUATOR Centre, member of the SPIRIT-CONSORT executive committee, director of Cochrane France and co-convenor of the Bias Methods group of the Cochrane Collaboration.

Dextromethorphan/bupropion (DXM/BUP), sold under the brand name Auvelity, is a combination medication for the treatment of major depressive disorder (MDD). Its active components are dextromethorphan (DXM) and bupropion. Patients who stayed on the medication had an average of 11% greater reduction in depressive symptoms than placebo in an FDA approval trial. It is taken as a tablet by mouth.

Alejandro R. Jadad Bechara is a Canadian-Colombian physician whose work focuses on creating a pandemic of health through creative human-machine collaboration powered by scientific evidence and collaboration across traditional boundaries. He is also known as the researcher responsible for the development of the Jadad Scale, the first validated tool to assess the methodological quality of clinical trials. He also co-created the methodology behind 'Computational Management', a systematic approach to facilitate task automation for the integration of artificial intelligence into existing workflows.
References
- 1 2 3 4 5 Jadad, A.R.; Moore R.A.; Carroll D.; Jenkinson C.; Reynolds D.J.M.; Gavaghan D.J.; McQuay H.J. (1996). "Assessing the quality of reports of randomized clinical trials: Is blinding necessary?". Controlled Clinical Trials. 17 (1): 1–12. doi:10.1016/0197-2456(95)00134-4. PMID 8721797.
- ↑ Jadad, Alejandro R.; Enkin, Murray (2007). Randomized Controlled Trials: Questions, Answers and Musings (2nd ed.). Blackwell. ISBN 978-1-4051-3266-4.
- 1 2 Chow, Shein-Chung; Liu, Jen-pei (2004). Design and Analysis of Clinical Trials. Wiley. p. 2. ISBN 978-0-471-24985-6.
- ↑ Brian, Everitt; Pickles, Andrew (2004). Statistical Aspects of the Design and Analysis of Clinical Trials. Imperial College Press. p. 5. ISBN 978-1-86094-441-3.
- 1 2 Colditz, G.A.; Miller J.N.; Mosteller F. (1989). "How study design affects outcomes in comparisons of therapy". Statistics in Medicine. 8 (4): 441–454. doi:10.1002/sim.4780080408. PMID 2727468.
- 1 2 3 Day, Simon J; Altman, Douglas G (2000). "Blinding in clinical trials and other studies". British Medical Journal. 321 (7259): 504. doi:10.1136/bmj.321.7259.504. PMC 1118396 . PMID 10948038.
- ↑ Altman, DG; Schulz, KF; Moher, D; Egger, M; Davidoff, F; Elbourne, D; Gøtzsche, PC; Lang, T; CONSORT GROUP (Consolidated Standards of Reporting Trials) (2001-04-17). "The revised CONSORT statement for reporting randomized trials: explanation and elaboration". Annals of Internal Medicine. 134 (8): 663–694. doi:10.7326/0003-4819-134-8-200104170-00012. PMID 11304107. S2CID 12834600.
- ↑ Lancaster T, Stead L (1999). Dealing with drop-outs in clinical trials and meta-analyses. 7th Best Evidence Health Care Cochrane Colloquium. Universita San Tommaso d'Aquino. p. 43.
- ↑ White, Adrian; Ernst, Edzard (1999). Acupuncture: A Scientific Appraisal. Elsevier. p. 109. ISBN 978-0-7506-4163-0.
- ↑ Wang, Gang; et al. (2007). "The quality of reporting of randomized controlled trials of traditional Chinese medicine". Clinical Therapeutics. 29 (7): 1456–1467. doi:10.1016/j.clinthera.2007.07.023. PMID 17825697.
- ↑ Welk, B.; Afshar, K.; MacNeily, A.E. (2006). "Randomized controlled trials in pediatric urology: room for improvement". J Urol. 176 (1): 306–310. doi:10.1016/S0022-5347(06)00560-X. PMID 16753430.
- ↑ Simon, Stephen D. (2006). Statistical Evidence in Medical Trials: What Do the Data Really Tell Us?. Oxford University Press. p. 122. ISBN 978-0-19-856761-5.
- ↑ Hayes, R.B.; Sackett, D.L.; Guyatt, G.H.; Tugwell, P. (2005). Clinical Epidemiology. Lippincott Williams & Wilkins. p. 31. ISBN 978-0-7817-4524-6.
- ↑ Olivo, SA; Macedo LG; Gadotti IC; Fuentes J; Stanton T; Magee DJ (2008). "Scales to Assess the Quality of Randomized Controlled Trials : A Systematic Review". Physical Therapy. 88 (2): 156–75. doi: 10.2522/ptj.20070147 . ISSN 0031-9023. PMID 18073267.
- ↑ Berger, V. W. (2006). "Is the Jadad Score the Proper Evaluation of Trials?". J. Rheumatol. 33 (8): 1710–1712. ISSN 0315-162X. PMID 16881132. Archived from the original on 2008-02-20.
- ↑ "Systematic Review of Quality Assessment Instruments for Randomized Control Trials". The Cochrane Collaboration. Retrieved 2008-11-12.
- ↑ Clark, H.D.; et al. (Oct 1999). "Assessing the quality of randomized trials: reliability of the Jadad scale". Controlled Clinical Trials . 20 (5): 448–52. doi:10.1016/S0197-2456(99)00026-4. PMID 10503804.
- ↑ Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.