Related Research Articles
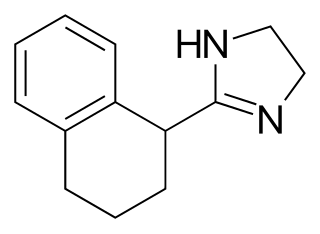
Tetryzoline is a drug used in some over-the-counter eye drops and nasal sprays. Tetryzoline was patented in 1954, and came into medical use in 1959.
A British Approved Name (BAN) is the official, non-proprietary, or generic name given to a pharmaceutical substance, as defined in the British Pharmacopoeia (BP). The BAN is also the official name used in some countries around the world, because starting in 1953, proposed new names were evaluated by a panel of experts from WHO in conjunction with the BP commission to ensure naming consistency worldwide (an effort leading to the International Nonproprietary Name system). There is also a British Approved Name (Modified) (BANM).
The British Pharmacopoeia (BP) is the national pharmacopoeia of the United Kingdom. It is an annually published collection of quality standards for medicinal substances in the UK, which is used by individuals and organisations involved in pharmaceutical research, development, manufacture and testing.

Thiotepa (INN), sold under the brand name Tepadina, is a medication used to treat cancer.

ISIS/Draw was a chemical structure drawing program developed by MDL Information Systems. It introduced a number of file formats for the storage of chemical information that have become industry standards.

Etoperidone, associated with several brand names, is an atypical antidepressant which was developed in the 1970s and either is no longer marketed or was never marketed. It is a phenylpiperazine related to trazodone and nefazodone in chemical structure and is a serotonin antagonist and reuptake inhibitor (SARI) similarly to them.

Martindale: The Complete Drug Reference is a reference book published by Pharmaceutical Press listing some 6,000 drugs and medicines used throughout the world, including details of over 125,000 proprietary preparations. It also includes almost 700 disease treatment reviews.

Cloperastine (INN) or cloperastin, in the forms of cloperastine hydrochloride (JAN) and cloperastine fendizoate, is an antitussive and antihistamine that is marketed as a cough suppressant in Japan, Hong Kong, and in some European countries. It was first introduced in 1972 in Japan, and then in Italy in 1981.
Aloglutamol is an antacid, an aluminium compound. It is a salt of aluminium, gluconic acid, and tris. It is usually given orally in doses of 0.5 to 1 g. Proprietary names include Altris, Pyreses, Tasto and Sabro.

Prasterone sulfate, also known as dehydroepiandrosterone sulfate (DHEA-S), is a naturally occurring androstane steroid which is marketed and used in Japan and other countries as a labor inducer in the treatment of insufficient cervical ripening and dilation during childbirth. It is the C3β sulfate ester of prasterone, and is known to act as a prohormone of DHEA and by extension of androgens and estrogens, although it also has its own activity as a neurosteroid. Prasterone sulfate is used medically as the sodium salt via injection and is referred to by the name sodium prasterone sulfate (JAN).

Oxaflozane (INN) (brand name Conflictan) is an antidepressant and anxiolytic drug that was introduced by Solvay in France in 1982 for the treatment of depression but has since been discontinued. It is a prodrug of flumexadol (N-dealkyloxaflozane; 2-(3-trifluoromethylphenyl)morpholine; CERM-1841 or 1841-CERM), which is reported to act as an agonist of the serotonin 5-HT1A (pKi = 7.1) and 5-HT2C (pKi = 7.5) receptors and, to a much lesser extent, of the 5-HT2A (pKi = 6.0) receptor. In addition to its serotonergic properties, oxaflozane may also produce anticholinergic side effects at high doses, namely in overdose.
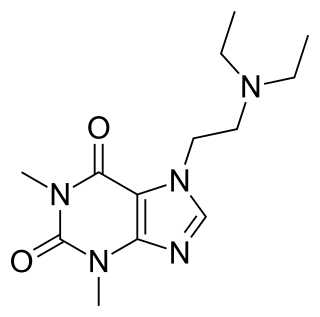
Etamiphylline or etamiphyllin (INN) is a xanthine intended for use as an anti-asthma agent. It has shown poor to absent effects in human clinical trials.

Homarylamine is an antitussive (anti-cough) drug which was patented in 1956 by Merck & Co., but has never been used medically as such.

Epitiostanol, sold under the brand name Thiodrol, is an injected antiestrogen and anabolic–androgenic steroid (AAS) of the dihydrotestosterone (DHT) group which was described in the literature in 1965 and has been marketed in Japan as an antineoplastic agent for the treatment of breast cancer since 1977.
Drug nomenclature is the systematic naming of drugs, especially pharmaceutical drugs. In the majority of circumstances, drugs have 3 types of names: chemical names, the most important of which is the IUPAC name; generic or nonproprietary names, the most important of which are international nonproprietary names (INNs); and trade names, which are brand names. Under the INN system, generic names for drugs are constructed out of affixes and stems that classify the drugs into useful categories while keeping related names distinguishable. A marketed drug might also have a company code or compound code.

Anagestone acetate, sold under the brand names Anatropin and Neo-Novum, is a progestin medication which was withdrawn from medical use due to carcinogenicity observed in animal studies.
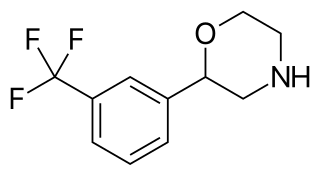
Flumexadol (INN) is a drug described and researched as a non-opioid analgesic which was never marketed. It has been found to act as an agonist of the serotonin 5-HT1A and 5-HT2C receptors and, to a much lesser extent, of the 5-HT2A receptor. According to Nilsson (2006) in a paper on 5-HT2C receptor agonists as potential anorectics, "The (+)-enantiomer of this compound showed [...] affinity for the 5-HT2C receptor (Ki) 25 nM) [...] and was 40-fold selective over the 5-HT2A receptor in receptor binding studies. Curiously, the racemic version [...], also known as 1841 CERM, was originally reported to possess analgesic properties while no association with 5-HT2C receptor activity was mentioned." It is implied that flumexadol might be employable as an anorectic in addition to analgesic. Though flumexadol itself has never been approved for medical use, oxaflozane is a prodrug of the compound that was formerly used clinically in France as an antidepressant and anxiolytic agent.

Atrimustine (INN), also known as bestrabucil or busramustine, is a cytostatic antineoplastic agent which was under development in Japan by Kureha Chemicals for the treatment of breast cancer and non-Hodgkin's lymphoma as well as for the prevention of graft-versus-host disease in bone marrow transplant recipients. It is the benzoate ester of an ester conjugate of estradiol and chlorambucil, which results in targeted/site-directed cytostatic activity toward estrogen receptor-positive tissues such as breast and bone. It reached preregistration for the treatment of cancer but was ultimately discontinued. Estrogenicic side effects of atrimustine in clinical trials included vaginal bleeding and gynecomastia. The drug was first patented in 1980.

Metenolone acetate, or methenolone acetate, sold under the brand names Primobolan and Nibal, is an androgen and anabolic steroid (AAS) medication which is used mainly in the treatment of anemia due to bone marrow failure. It is taken by mouth. Although it was widely used in the past, the drug has mostly been discontinued and hence is now mostly no longer available. A related drug, metenolone enanthate, is given by injection into muscle.

Monalazone, used as monalazone disodium and sold under the brand names Naclobenz-Natrium, Spergisin, and Speton, is a vaginal disinfectant or antiseptic and spermicidal contraceptive. It is a sulfonylbenzoic acid derivative and is closely related structurally to halazone. The compound was synthesized in 1937. A vaginal tablet combination of 0.125 mg estradiol benzoate and 10 mg monalazone was previously marketed under the brand name Malun 25.
References
- ↑ Croswell, Roger; Cartwright, Anthony C. (2010). "3. CTD Module 1 - Administrative information". In Cartwright, Anthony C.; Matthews, Brian R. (eds.). International Pharmaceutical Product Registration (2nd ed.). CRC Press. p. 31. ISBN 978-1-4200-8183-1.
- ↑ Senning, Alexander (2019). "14. International non-proprietary names for drugs and excipients". The Etymology of Chemical Names: Tradition and Convenience vs. Rationality in Chemical Nomenclature. Denmark: De Gruyter. p. 413. ISBN 978-3-11-061276-9.