
Cold fusion is a hypothesized type of nuclear reaction that would occur at, or near, room temperature. It would contrast starkly with the "hot" fusion that is known to take place naturally within stars and artificially in hydrogen bombs and prototype fusion reactors under immense pressure and at temperatures of millions of degrees, and be distinguished from muon-catalyzed fusion. There is currently no accepted theoretical model that would allow cold fusion to occur.
Bobby Stanley Pons is an American electrochemist known for his work with Martin Fleischmann on cold fusion in the 1980s and 1990s.
Revaz Dogonadze was a notable Georgian scientist, Corresponding Member of the Georgian National Academy of Sciences (GNAS) (1982), Doctor of Physical & Mathematical Sciences (1966), Professor (1972), one of the founders of Quantum electrochemistry,

A reference electrode is an electrode that has a stable and well-known electrode potential. The overall chemical reaction taking place in a cell is made up of two independent half-reactions, which describe chemical changes at the two electrodes. To focus on the reaction at the working electrode, the reference electrode is standardized with constant concentrations of each participant of the redox reaction.
Bernhardt Patrick John O’Mara Bockris was a South African professor of chemistry, latterly at Texas A&M University. During his long and prolific career he published some 700 papers and two dozen books. His best known work is in electrochemistry but his output also extended to environmental chemistry, photoelectrochemistry and bioelectrochemistry. In the 1990s he experimented with cold fusion and transmutation, topics on which his unorthodox views provoked controversy.
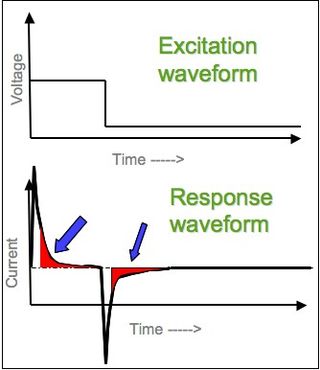
In electrochemistry, chronoamperometry is an analytical technique in which the electric potential of the working electrode is stepped and the resulting current from faradaic processes occurring at the electrode is monitored as a function of time. The functional relationship between current response and time is measured after applying single or double potential step to the working electrode of the electrochemical system. Limited information about the identity of the electrolyzed species can be obtained from the ratio of the peak oxidation current versus the peak reduction current. However, as with all pulsed techniques, chronoamperometry generates high charging currents, which decay exponentially with time as any RC circuit. The Faradaic current - which is due to electron transfer events and is most often the current component of interest - decays as described in the Cottrell equation. In most electrochemical cells, this decay is much slower than the charging decay-cells with no supporting electrolyte are notable exceptions. Most commonly a three-electrode system is used. Since the current is integrated over relatively longer time intervals, chronoamperometry gives a better signal-to-noise ratio in comparison to other amperometric techniques.
Meitnerium (109Mt) is a synthetic element, and thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 266Mt in 1982, and this is also the only isotope directly synthesized; all other isotopes are only known as decay products of heavier elements. There are eight known isotopes, from 266Mt to 278Mt. There may also be two isomers. The longest-lived of the known isotopes is 278Mt with a half-life of 8 seconds. The unconfirmed heavier 282Mt appears to have an even longer half-life of 67 seconds.
Roentgenium (111Rg) is a synthetic element, and thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 272Rg in 1994, which is also the only directly synthesized isotope; all others are decay products of heavier elements. There are seven known radioisotopes, having mass numbers of 272, 274, and 278–282. The longest-lived isotope is 282Rg with a half-life of about 2 minutes, although the unconfirmed 283Rg and 286Rg may have longer half-lives of about 5.1 minutes and 10.7 minutes respectively.
Nihonium (113Nh) is a synthetic element. Being synthetic, a standard atomic weight cannot be given and like all artificial elements, it has no stable isotopes. The first isotope to be synthesized was 284Nh as a decay product of 288Mc in 2003. The first isotope to be directly synthesized was 278Nh in 2004. There are 6 known radioisotopes from 278Nh to 286Nh, along with the unconfirmed 287Nh and 290Nh. The longest-lived isotope is 286Nh with a half-life of 9.5 seconds.
A solvated electron is a free electron in a solution, and is the smallest possible anion. Solvated electrons occur widely. Often, discussions of solvated electrons focus on their solutions in ammonia, which are stable for days, but solvated electrons also occur in water and other solvents – in fact, in any solvent that mediates outer-sphere electron transfer. The solvated electron is responsible for a great deal of radiation chemistry.

Martin Fleischmann FRS was a British chemist who worked in electrochemistry. Premature announcement of his cold fusion research with Stanley Pons, regarding excess heat in heavy water, caused a media sensation and elicited skepticism and criticism from many in the scientific community.
Water oxidation is one of the half reactions of water splitting:
A liquid metal electrode is an electrode that uses a liquid metal, such as mercury, Galinstan, and NaK. They can be used in electrocapillarity, voltammetry, and impedance measurements.

The Journal of Colloid and Interface Science is a peer-reviewed scientific journal published by Elsevier. It covers research related to colloid and interface science with a particular focus on colloidal materials and nanomaterials; surfactants and soft matter; adsorption, catalysis and electrochemistry; interfacial processes, capillarity and wetting; biomaterials and nanomedicine; and novel phenomena and techniques. The editor-in-chief is Martin Malmsten. The journal was established in 1946 as Journal of Colloid Science. It obtained its current name in 1966.

Andrzej Wieckowski was an Emeritus Professor of Chemistry at the University of Illinois at Urbana–Champaign and the North American Editor of Electrochimica Acta. He is known for his spectroscopic investigations of electrocatalysis in fuel cells and co-inventing of the direct formic acid fuel cell (DFAFC). He authored more than 300 publications, has been cited over 13,000 times and has an h-index 60. He was appointed fellow of the Electrochemical Society in 2007 and fellow of the International Society of Electrochemistry in 2009. He was awarded the US Department of Energy Prize for outstanding Scientific Accomplishment in Materials Chemistry in 1992, the ISE Jacques Tacussel Prize in 1998, the ECS David. C. Graham Award in 2003, and the ISE Gold Medal in 2007.
In electrochemistry, protein film voltammetry is a technique for examining the behavior of proteins immobilized on an electrode. The technique is applicable to proteins and enzymes that engage in electron transfer reactions and it is part of the methods available to study enzyme kinetics.

Electrochemical quartz crystal microbalance (EQCM) is the combination of electrochemistry and quartz crystal microbalance, which was generated in the eighties. Typically, an EQCM device contains an electrochemical cells part and a QCM part. Two electrodes on both sides of the quartz crystal serve two purposes. Firstly, an alternating electric field is generated between the two electrodes for making up the oscillator. Secondly, the electrode contacting electrolyte is used as a working electrode (WE), together with a counter electrode (CE) and a reference electrode (RE), in the potentiostatic circuit constituting the electrochemistry cell. Thus, the working electrode of electrochemistry cell is the sensor of QCM.
Janet Gretchen Osteryoung was an American chemist who was the director of the Chemistry Division of the National Science Foundation from 1994 to 2001. Her research furthered the development of electroanalysis and especially that of square wave voltammetry. She was elected a Fellow of the American Association for the Advancement of Science in 1984 and awarded the Garvan–Olin Medal in 1987.

Hydrogen ozonide is a radical molecule consisting of a hydrogen atom covalently bonded to an ozonide unit.

Theodore Kuwana (1931–2022) was a chemist and academic researcher known as the founding father of the field of spectroelectrochemistry.









