Related Research Articles

Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer (passivation) somewhat stabilizes the free metal against further oxidation.

Ilmenite, also known as manaccanite, is a titanium-iron oxide mineral with the idealized formula FeTiO
3. It is a weakly magnetic black or steel-gray solid. From a commercial perspective, ilmenite is the most important ore of titanium. Ilmenite is the main source of titanium dioxide, which is used in paints, printing inks, fabrics, plastics, paper, sunscreen, food and cosmetics.

Vanadinite is a mineral belonging to the apatite group of phosphates, with the chemical formula Pb5(VO4)3Cl. It is one of the main industrial ores of the metal vanadium and a minor source of lead. A dense, brittle mineral, it is usually found in the form of red hexagonal crystals. It is an uncommon mineral, formed by the oxidation of lead ore deposits such as galena. First discovered in 1801 in Mexico, vanadinite deposits have since been unearthed in South America, Europe, Africa, and North America.

Carnotite is a potassium uranium vanadate radioactive mineral with chemical formula K2(UO2)2(VO4)2·3H2O. The water content can vary and small amounts of calcium, barium, magnesium, iron, and sodium are often present.

Group 5 is a group of elements in the periodic table. Group 5 contains vanadium (V), niobium (Nb), tantalum (Ta) and dubnium (Db). This group lies in the d-block of the periodic table. The group itself has not acquired a trivial name; it belongs to the broader grouping of the transition metals.

Vanabins are a specific group of vanadium-binding metalloproteins. Vanabins are found almost exclusively in the blood cells, or vanadocytes, of some tunicates, including the Ascidiacea. The vanabins extracted from tunicate vanadocytes are often called hemovanadins. These organisms are able to concentrate vanadium to a level more than, and sometimes much more than, 100 times higher than in the surrounding seawater. Vanabin proteins seem to be involved in collecting and accumulating this metal ion. At present there is no conclusive understanding of why these organisms collect vanadium.
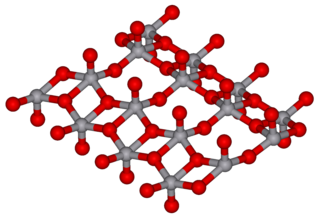
Vanadium(V) oxide (vanadia) is the inorganic compound with the formula V2O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst.

Volborthite is a mineral containing copper and vanadium, with the formula Cu3V2O7(OH)2·2H2O. Found originally in 1838 in the Urals, it was first named knaufite but was later changed to volborthite for Alexander von Volborth (1800–1876), a Russian paleontologist.

Sodium metavanadate is the inorganic compound with the formula NaVO3. It is a yellow, water-soluble salt.

Vanadium(III) oxide is the inorganic compound with the formula V2O3. It is a black solid prepared by reduction of V2O5 with hydrogen or carbon monoxide. It is a basic oxide dissolving in acids to give solutions of vanadium (III) complexes. V2O3 has the corundum structure. It is antiferromagnetic with a critical temperature of 160 K. At this temperature there is an abrupt change in conductivity from metallic to insulating.

Vanadium(II) oxide, VO, is one of the many oxides of vanadium. VO is a long-lived, electronically neutral reagent chemical. It adopts a distorted NaCl structure and contains weak V−V metal to metal bonds. As shown by band theory, VO is a conductor of electricity due to its partially filled conduction band and delocalisation of electrons in the t2g orbitals. VO is a non-stoichiometric compound, its composition varying from VO0.8 to VO1.3. Gaseous VO is one of the molecules found in the spectrum of relatively cool M-type stars.

Vanadyl(IV) sulfate describes a collection of inorganic compounds of vanadium with the formula, VOSO4(H2O)x where 0 ≤ x ≤ 6. The pentahydrate is common. This hygroscopic blue solid is one of the most common sources of vanadium in the laboratory, reflecting its high stability. It features the vanadyl ion, VO2+, which has been called the "most stable diatomic ion".
Vanadium oxide may refer to:

Uranium mining in Colorado, United States, goes back to 1872, when pitchblende ore was taken from gold mines near Central City, Colorado. The Colorado uranium industry has seen booms and busts, but continues to this day. Not counting byproduct uranium from phosphate, Colorado is considered to have the third largest uranium reserves of any US state, behind Wyoming and New Mexico.

Roscoelite is a green mineral from the mica group that contains vanadium.

Uranium mining in Utah, a state of the United States, has a history going back more than 100 years. Uranium mining started as a byproduct of vanadium mining about 1900, became a byproduct of radium mining about 1910, then back to a byproduct of vanadium when the radium price fell in the 1920s. Utah saw a uranium boom in the late 1940s and early 1950s, but uranium mining declined in the 1980s. Since 2001 there has been a revival of interest in uranium mining, as a result of higher uranium prices.
Schreyerite (V2Ti3O9), is a vanadium, titanium oxide mineral found in the Lasamba Hill, Kwale district in Coast Province, Kenya. It is polymorphous with kyzylkumite.

Patronite is the vanadium sulfide mineral with formula VS4. The material is usually described as V4+(S22−)2. Structurally, it is a "linear-chain" compound with alternating bonding and nonbonding contacts between the vanadium centers. The vanadium is octa-coordinated, which is an uncommon geometry for this metal.
Tistarite is an exceedingly rare mineral with the formula Ti2O3, thus being the natural analogue of titanium(III) oxide. In terms of chemistry it is the titanium-analogue of hematite, corundum, eskolaite, and karelianite. Other minerals with the general formula A2O3 are arsenolite, avicennite, claudetite, bismite, bixbyite, kangite, sphaerobismoite, yttriaite-(Y) and valentinite. Tistarite and grossmanite - both found in the famous Allende meteorite (so is kangite) - are the only currently known minerals with trivalent titanium. Titanium in minerals is almost exclusively tetravalent. The only known terrestrial occurrence of tistarite was found during minerals exploration by Shefa Yamim Ltd. in the upper mantle beneath Mount Carmel, Israel.
Native vanadium is the mineral form of the metal vanadium, with the accepted name "vanadium". This exceedingly rare mineral was found among fumaroles of the Colima volcano. Fumaroles of Colima are known of being vanadium-rich, depositing other vanadium minerals, that include shcherbinaite (V2O5) and colimaite (K3VS4).
References
- ↑ "Karelianite: Mineral information, data and localities". www.mindat.org. Retrieved 2019-04-10.