
The photoelectric effect is the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, solid state, and quantum chemistry to draw inferences about the properties of atoms, molecules and solids. The effect has found use in electronic devices specialized for light detection and precisely timed electron emission.

In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or a material medium. This includes:

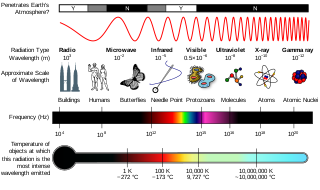
X-ray is a high-energy electromagnetic radiation. In many languages, it is referred to as Röntgen radiation, after the German scientist Wilhelm Conrad Röntgen, who discovered it in 1895 and named it X-radiation to signify an unknown type of radiation.

A beta particle, also called beta ray or beta radiation, is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β− decay and β+ decay, which produce electrons and positrons respectively.

In particle physics, bremsstrahlung is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into radiation, thus satisfying the law of conservation of energy. The term is also used to refer to the process of producing the radiation. Bremsstrahlung has a continuous spectrum, which becomes more intense and whose peak intensity shifts toward higher frequencies as the change of the energy of the decelerated particles increases.

Acute radiation syndrome (ARS), also known as radiation sickness or radiation poisoning, is a collection of health effects that are caused by being exposed to high amounts of ionizing radiation in a short period of time. Symptoms can start within an hour of exposure, and can last for several months. Early symptoms are usually nausea, vomiting and loss of appetite. In the following hours or weeks, initial symptoms may appear to improve, before the development of additional symptoms, after which either recovery or death follow.

The sievert is a unit in the International System of Units (SI) intended to represent the stochastic health risk of ionizing radiation, which is defined as the probability of causing radiation-induced cancer and genetic damage. The sievert is important in dosimetry and radiation protection. It is named after Rolf Maximilian Sievert, a Swedish medical physicist renowned for work on radiation dose measurement and research into the biological effects of radiation.
Ionizing radiation, including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel up to 99% of the speed of light, and the electromagnetic waves are on the high-energy portion of the electromagnetic spectrum.

External beam radiation therapy (EBRT) is a form of radiotherapy that utilizes a high-energy collimated beam of ionizing radiation, from a source outside the body, to target and kill cancer cells. A radiotherapy beam is composed of particles which travel in a consistent direction; each radiotherapy beam consists of one type of particle intended for use in treatment, though most beams contain some contamination by other particle types.
The gray is the unit of ionizing radiation dose in the International System of Units (SI), defined as the absorption of one joule of radiation energy per kilogram of matter.
Radiation dosimetry in the fields of health physics and radiation protection is the measurement, calculation and assessment of the ionizing radiation dose absorbed by an object, usually the human body. This applies both internally, due to ingested or inhaled radioactive substances, or externally due to irradiation by sources of radiation.
Radiation protection, also known as radiological protection, is defined by the International Atomic Energy Agency (IAEA) as "The protection of people from harmful effects of exposure to ionizing radiation, and the means for achieving this". Exposure can be from a source of radiation external to the human body or due to internal irradiation caused by the ingestion of radioactive contamination.

Health physics, also referred to as the science of radiation protection, is the profession devoted to protecting people and their environment from potential radiation hazards, while making it possible to enjoy the beneficial uses of radiation. Health physicists normally require a four-year bachelor’s degree and qualifying experience that demonstrates a professional knowledge of the theory and application of radiation protection principles and closely related sciences. Health physicists principally work at facilities where radionuclides or other sources of ionizing radiation are used or produced; these include research, industry, education, medical facilities, nuclear power, military, environmental protection, enforcement of government regulations, and decontamination and decommissioning—the combination of education and experience for health physicists depends on the specific field in which the health physicist is engaged.
Absorbed dose is a dose quantity which is the measure of the energy deposited in matter by ionizing radiation per unit mass. Absorbed dose is used in the calculation of dose uptake in living tissue in both radiation protection, and radiology. It is also used to directly compare the effect of radiation on inanimate matter such as in radiation hardening.
The Röntgen equivalent physical or rep is a legacy unit of absorbed dose first introduced by Herbert Parker in 1945 to replace an improper application of the roentgen unit to biological tissue. It is the absorbed energetic dose before the biological efficiency of the radiation is factored in. The rep has variously been defined as 83 or 93 ergs per gram of tissue (8.3/9.3 mGy) or per cm3 of tissue.

In dosimetry, linear energy transfer (LET) is the amount of energy that an ionizing particle transfers to the material traversed per unit distance. It describes the action of radiation into matter.
In radiobiology, the relative biological effectiveness is the ratio of biological effectiveness of one type of ionizing radiation relative to another, given the same amount of absorbed energy. The RBE is an empirical value that varies depending on the type of ionizing radiation, the energies involved, the biological effects being considered such as cell death, and the oxygen tension of the tissues or so-called oxygen effect.

A gamma ray, also known as gamma radiation (symbol γ or ), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz (3×1019 Hz), it imparts the highest photon energy. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation gamma rays based on their relatively strong penetration of matter; in 1900 he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power.

The roentgen or röntgen is a legacy unit of measurement for the exposure of X-rays and gamma rays, and is defined as the electric charge freed by such radiation in a specified volume of air divided by the mass of that air . In 1928, it was adopted as the first international measurement quantity for ionizing radiation to be defined for radiation protection, as it was then the most easily replicated method of measuring air ionization by using ion chambers. It is named after the German physicist Wilhelm Röntgen, who discovered X-rays and was awarded the first Nobel Prize in Physics for the discovery.
Radiation exposure is a measure of the ionization of air due to ionizing radiation from photons. It is defined as the electric charge freed by such radiation in a specified volume of air divided by the mass of that air. As of 2007, "medical radiation exposure" was defined by the International Commission on Radiological Protection as exposure incurred by people as part of their own medical or dental diagnosis or treatment; by persons, other than those occupationally exposed, knowingly, while voluntarily helping in the support and comfort of patients; and by volunteers in a programme of biomedical research involving their exposure. Common medical tests and treatments involving radiation include X-rays, CT scans, mammography, lung ventilation and perfusion scans, bone scans, cardiac perfusion scan, angiography, radiation therapy, and more. Each type of test carries its own amount of radiation exposure. There are two general categories of adverse health effects caused by radiation exposure: deterministic effects and stochastic effects. Deterministic effects are due to the killing/malfunction of cells following high doses; and stochastic effects involve either cancer development in exposed individuals caused by mutation of somatic cells, or heritable disease in their offspring from mutation of reproductive (germ) cells.

















