
The Zeeman effect is the effect of splitting of a spectral line into several components in the presence of a static magnetic field. It is named after the Dutch physicist Pieter Zeeman, who discovered it in 1896 and received a Nobel prize for this discovery. It is analogous to the Stark effect, the splitting of a spectral line into several components in the presence of an electric field. Also similar to the Stark effect, transitions between different components have, in general, different intensities, with some being entirely forbidden, as governed by the selection rules.
In atomic, molecular, and solid-state physics, the electric field gradient (EFG) measures the rate of change of the electric field at an atomic nucleus generated by the electronic charge distribution and the other nuclei. The EFG couples with the nuclear electric quadrupole moment of quadrupolar nuclei to generate an effect which can be measured using several spectroscopic methods, such as nuclear magnetic resonance (NMR), microwave spectroscopy, electron paramagnetic resonance, nuclear quadrupole resonance (NQR), Mössbauer spectroscopy or perturbed angular correlation (PAC). The EFG is non-zero only if the charges surrounding the nucleus violate cubic symmetry and therefore generate an inhomogeneous electric field at the position of the nucleus.

In atomic physics, hyperfine structure is defined by small shifts in otherwise degenerate energy levels and the resulting splittings in those energy levels of atoms, molecules, and ions, due to electromagnetic multipole interaction between the nucleus and electron clouds.
Nuclear quadrupole resonance spectroscopy or NQR is a chemical analysis technique related to nuclear magnetic resonance (NMR). Unlike NMR, NQR transitions of nuclei can be detected in the absence of a magnetic field, and for this reason NQR spectroscopy is referred to as "zero Field NMR". The NQR resonance is mediated by the interaction of the electric field gradient (EFG) with the quadrupole moment of the nuclear charge distribution. Unlike NMR, NQR is applicable only to solids and not liquids, because in liquids the electric field gradient at the nucleus averages to zero. Because the EFG at the location of a nucleus in a given substance is determined primarily by the valence electrons involved in the particular bond with other nearby nuclei, the NQR frequency at which transitions occur is unique for a given substance. A particular NQR frequency in a compound or crystal is proportional to the product of the nuclear quadrupole moment, a property of the nucleus, and the EFG in the neighborhood of the nucleus. It is this product which is termed the nuclear quadrupole coupling constant for a given isotope in a material and can be found in tables of known NQR transitions. In NMR, an analogous but not identical phenomenon is the coupling constant, which is also the result of an internuclear interaction between nuclei in the analyte.
Dynamic nuclear polarization (DNP) results from transferring spin polarization from electrons to nuclei, thereby aligning the nuclear spins to the extent that electron spins are aligned. Note that the alignment of electron spins at a given magnetic field and temperature is described by the Boltzmann distribution under the thermal equilibrium. It is also possible that those electrons are aligned to a higher degree of order by other preparations of electron spin order such as: chemical reactions, optical pumping and spin injection. DNP is considered one of several techniques for hyperpolarization. DNP can also be induced using unpaired electrons produced by radiation damage in solids.
RKKY stands for Ruderman–Kittel–Kasuya–Yosida. It refers to a coupling mechanism of nuclear magnetic moments or localized inner d- or f-shell electron spins in a metal by means of an interaction through the conduction electrons.
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure of a molecule. Chemical shifts are also used to describe signals in other forms of spectroscopy such as photoemission spectroscopy.
In quantum physics, the spin–orbit interaction is a relativistic interaction of a particle's spin with its motion inside a potential. A key example of this phenomenon is the spin–orbit interaction leading to shifts in an electron's atomic energy levels, due to electromagnetic interaction between the electron's magnetic dipole, its orbital motion, and the electrostatic field of the positively charged nucleus. This phenomenon is detectable as a splitting of spectral lines, which can be thought of as a Zeeman effect product of two relativistic effects: the apparent magnetic field seen from the electron perspective and the magnetic moment of the electron associated with its intrinsic spin. A similar effect, due to the relationship between angular momentum and the strong nuclear force, occurs for protons and neutrons moving inside the nucleus, leading to a shift in their energy levels in the nucleus shell model. In the field of spintronics, spin–orbit effects for electrons in semiconductors and other materials are explored for technological applications. The spin–orbit interaction is at the origin of magnetocrystalline anisotropy and the spin Hall effect.

Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. This spectroscopy is based on the measurement of absorption of electromagnetic radiations in the radio frequency region from roughly 4 to 900 MHz. Absorption of radio waves in the presence of magnetic field is accompanied by a special type of nuclear transition, and for this reason, such type of spectroscopy is known as Nuclear Magnetic Resonance Spectroscopy. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds.

Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spins excited are those of the electrons instead of the atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes and organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford.
In physics, Larmor precession is the precession of the magnetic moment of an object about an external magnetic field. The phenomenon is conceptually similar to the precession of a tilted classical gyroscope in an external torque-exerting gravitational field. Objects with a magnetic moment also have angular momentum and effective internal electric current proportional to their angular momentum; these include electrons, protons, other fermions, many atomic and nuclear systems, as well as classical macroscopic systems. The external magnetic field exerts a torque on the magnetic moment,
Ferromagnetic resonance, or FMR, is coupling between an electromagnetic wave and the magnetization of a medium through which it passes. This coupling induces a significant loss of power of the wave. The power is absorbed by the precessing magnetization of the material and lost as heat. For this coupling to occur, the frequency of the incident wave must be equal to the precession frequency of the magnetization and the polarization of the wave must match the orientation of the magnetization.
In MRI and NMR spectroscopy, an observable nuclear spin polarization (magnetization) is created by a homogeneous magnetic field. This field makes the magnetic dipole moments of the sample precess at the resonance (Larmor) frequency of the nuclei. At thermal equilibrium, nuclear spins precess randomly about the direction of the applied field. They become abruptly phase coherent when they are hit by radiofrequency (RF) pulses at the resonant frequency, created orthogonal to the field. The RF pulses cause the population of spin-states to be perturbed from their thermal equilibrium value. The generated transverse magnetization can then induce a signal in an RF coil that can be detected and amplified by an RF receiver. The return of the longitudinal component of the magnetization to its equilibrium value is termed spin-latticerelaxation while the loss of phase-coherence of the spins is termed spin-spin relaxation, which is manifest as an observed free induction decay (FID).

Zero- to ultralow-field (ZULF) NMR is the acquisition of nuclear magnetic resonance (NMR) spectra of chemicals with magnetically active nuclei in an environment carefully screened from magnetic fields. ZULF NMR experiments typically involve the use of passive or active shielding to attenuate Earth’s magnetic field. This is in contrast to the majority of NMR experiments which are performed in high magnetic fields provided by superconducting magnets. In ZULF experiments the dominant interactions are nuclear spin-spin couplings, and the coupling between spins and the external magnetic field is a perturbation to this. There are a number of advantages to operating in this regime: magnetic-susceptibility-induced line broadening is attenuated which reduces inhomogeneous broadening of the spectral lines for samples in heterogeneous environments. Another advantage is that the low frequency signals readily pass through conductive materials such as metals due to the increased skin depth; this is not the case for high-field NMR for which the sample containers are usually made of glass, quartz or ceramic.
Spin is an intrinsic form of angular momentum carried by elementary particles, and thus by composite particles such as hadrons, atomic nuclei, and atoms. Spin should not be understood as in the "rotating internal mass" sense: spin is a quantized wave property.

Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI).
Electron nuclear double resonance (ENDOR) is a magnetic resonance technique for elucidating the molecular and electronic structure of paramagnetic species. The technique was first introduced to resolve interactions in electron paramagnetic resonance (EPR) spectra. It is currently practiced in a variety of modalities, mainly in the areas of biophysics and heterogeneous catalysis.

The physics of magnetic resonance imaging (MRI) concerns fundamental physical considerations of MRI techniques and technological aspects of MRI devices. MRI is a medical imaging technique mostly used in radiology and nuclear medicine in order to investigate the anatomy and physiology of the body, and to detect pathologies including tumors, inflammation, neurological conditions such as stroke, disorders of muscles and joints, and abnormalities in the heart and blood vessels among others. Contrast agents may be injected intravenously or into a joint to enhance the image and facilitate diagnosis. Unlike CT and X-ray, MRI uses no ionizing radiation and is, therefore, a safe procedure suitable for diagnosis in children and repeated runs. Patients with specific non-ferromagnetic metal implants, cochlear implants, and cardiac pacemakers nowadays may also have an MRI in spite of effects of the strong magnetic fields. This does not apply on older devices, and details for medical professionals are provided by the device's manufacturer.
Magnetochemistry is concerned with the magnetic properties of chemical compounds. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons. Molecular compounds that contain one or more unpaired electrons are paramagnetic. The magnitude of the paramagnetism is expressed as an effective magnetic moment, μeff. For first-row transition metals the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula. In general, spin–orbit coupling causes μeff to deviate from the spin-only formula. For the heavier transition metals, lanthanides and actinides, spin–orbit coupling cannot be ignored. Exchange interaction can occur in clusters and infinite lattices, resulting in ferromagnetism, antiferromagnetism or ferrimagnetism depending on the relative orientations of the individual spins.
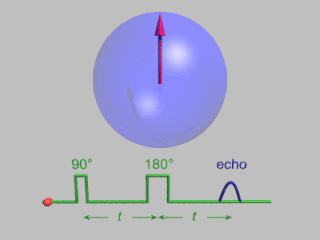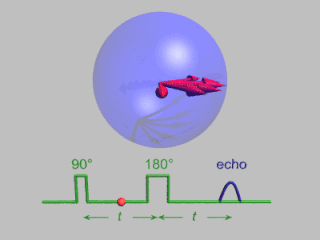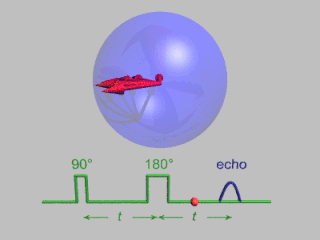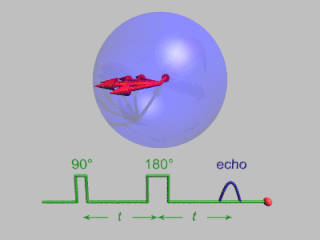
Pulsed electron paramagnetic resonance (EPR) is an electron paramagnetic resonance technique that involves the alignment of the net magnetization vector of the electron spins in a constant magnetic field. This alignment is perturbed by applying a short oscillating field, usually a microwave pulse. One can then measure the emitted microwave signal which is created by the sample magnetization. Fourier transformation of the microwave signal yields an EPR spectrum in the frequency domain. With a vast variety of pulse sequences it is possible to gain extensive knowledge on structural and dynamical properties of paramagnetic compounds. Pulsed EPR techniques such as electron spin echo envelope modulation (ESEEM) or pulsed electron nuclear double resonance (ENDOR) can reveal the interactions of the electron spin with its surrounding nuclear spins.












