
A LO NOx burner is a type of burner that is typically used in utility boilers to produce steam and electricity.

A LO NOx burner is a type of burner that is typically used in utility boilers to produce steam and electricity.

Around 1986 John Joyce (of Bowin Cars fame), an influential Australian inventor, first learned about oxides of nitrogen (NOx) and their role in the production of smog and acid rain. His first introduction to the complexities of the subject was brought about by the work of Fred Barnes and Dr John Bromley from the state Energy Commission of Western Australia. [1]
The vast majority of the research and development stretching back over twenty years was about large scale industrial burners and complex mechanisms which, in the end, did not produce what one would consider low NOx (2 ng/J or ~ 4 ppm at 0% O2 on dry basis). [2]
In fact at that time, 15 ng/J NO2 appears to have been considered low NO2. The one clear message that did flow through all the mass of information he studied, was the effect of temperature on the formation of NOx.
In the late 1980s, Health and Environment Authorities in Australia raised concerns about the indoor air quality and the extent that particularly older style unflued gas heaters were contributing to higher than acceptable levels of nitrogen dioxide (NO2). Consequently in 1989 the New South Wales Department of School Education initiated an extensive investigation of nitrogen dioxide in schools throughout New South Wales. As an interim measure the Health Authorities advised that a level of 0.3 ppm NO2 should become the upper limit for classrooms. [3] The Australian Gas Association in turn reduced the indoor emission rate of NO2 for unflued gas heaters from 15 to 5 ng/J and this remains the current limit. [4] The New South Wales government, through the Public Works Department, also re-evaluated alternative methods of heating classrooms, to ensure a safe and healthy environment for students.
It was in this context, that John Joyce's company Bowin Technology embarked on a major research & development program aimed at minimising nitrogen dioxide emissions from unflued gas heaters. Bowin Technology set itself the task of solving the emission problem at its source: the gas burner. This was despite a generally long held belief by gas experts, that commercially warranted gas burner improvements could not deliver drastic nitrogen oxides (NOx) reductions.
In 1989, an immediate call to reduce the indoor nitrogen dioxide (NO2) level, was triggered by widely publicised articles and media coverage in New South Wales, highlighting the effect this chemical has on respiratory sensitive people, such as asthmatics and those with bronchial problems.
In the heat of the indoor air quality debate various State institutions in Australia were advised to switch to flued gas heaters and electric heating.
New South Wales in contrast, through combined action by Australian Gas Light Company, Health Authorities and the New South Wales Public Works Department, formulated initial indoor air quality guidelines. These guidelines formed the basis for Australian Gas Appliance Code restrictions for nitrogen dioxide NO2emissions from unflued heaters, now adopted Australia wide. [4]
John Joyce became aware that no other overseas regulatory body made a distinction between NO and NO2 in their environmental guidelines or codes. Furthermore it appeared that total nitrogen oxides level requirements were in place irrespective of whether emissions were flued or not.
Consequently John Joyce learned that a 'harmless' part of NOx emissions, nitric oxide (NO), in the presence of hydrocarbons (such as household aerosol propellants, possible gas leaks and ingress of vehicle exhaust fume), converts to NO2. This was found to be the case in the New South Wales school investigation. [3] In a scientific sense it had become practice to calculate both NO + NO2, when measuring oxides of nitrogen levels in emissions. Hence the now commonly used reference to "total NOx".
Natural gas by composition has a distinct advantage over other fossil fuels in terms of carbon dioxide, particulate and sulfur dioxides produced when converting to useful energy. In the early 1990s numerous countries were in the process of substituting oil and coal with natural gas for their energy and electric power needs.
To maintain this advantage as an "environmentally friendly" fuel, Australian gas utilities are effectively reducing gas losses (methane emissions) in their deliveries, and impose strict codes on appliance manufacturers and installers against gas leakage.
Nevertheless environmental experts see the production of oxides of nitrogen as a major menace in the formation of greenhouse gases and photochemical smog. The interaction of NOx with hydrocarbons from vehicle exhausts and sunlight can also form low level ozone. In the stratosphere (some 25 km up), ozone is helpful by absorbing the fiercer part of the ultraviolet radiation of the sun, but at ground level it damages materials and vegetation. It irritates throat, lungs and eyes, and strenuous exercise or work can become painful. Furthermore, the effectiveness of nitrous oxide as a greenhouse gas is magnified by its longer life than carbon dioxide, methane and CFC's.
In essence the rate at which low level ozone is formed is determined by hydrocarbons, whilst the availability of oxides of nitrogen influences the amount it produces. At this point the environmental debate takes a surprising turn as individual industries tend to blame each other's emission as a probable cause.
It is well established that conventional "blue flame" or bunsen gas burners produce oxides of nitrogen at levels of 30-50 nanograms per joule [5] [6] and are as such not considered to have potential for NOx reduction. Surface combustion burners or radiant tile burners in comparison produce nitrogen oxides' levels 60-70% less. [6] Therefore John Joyce's research into low NOx burners revolved primarily around surface combustion techniques. Another issue was the effect combustion temperatures have on the formation of NOx.
John Joyce's task became even more challenging when he decided not to direct his development towards radiant type surface combustion tiles. The use of radiant heating for most institutional purposes (other than spot heating) is considered impractical as is too hot close to the heater, while the loss of radiant heat over a distance to be reached is quite dramatic.
Investigations into numerous developments of other types of "low NOx" burners showed that so far such burners were either too complex in design or operation, too expensive or unsuitable. John Joyce's plan was to use high temperature steel mesh, and went on to produce scores of prototype burners until one showed "potential".
The scientific innovative nature of John Joyce's LO-NOx technologies are confirmed by full patent protection in Australia, United States, United Kingdom, Japan, Italy and France.
In 1993 John Joyce received an Australian Design Award and Powerhouse Museum Selection status for his "SLE" heater range, which incorporate LO-NOx burners.
The Australian Academy of Design selected the SLE unflued gas heater range to be featured in the Design Showcase during the "Innovation by Design" National Conference in October 1994
In the United States, John Joyce's LO-NOx water heater burners have successfully undergone a series of exhaustive tests to prove that these particular burners do not act as an ignition source in the presence of flammable vapours, resulting from accidental fuel spillage. There have also been extensive tests carried out to verify its reduction of NO2.
More tangible cost savings are defined when comparing the energy efficiencies of gas heaters with low NOx emissions with conventional flued types. Gas heaters with emission problems are flued and inherently lose substantial energies in the form of hot flue gases to the atmosphere. In addition, the choice of placement of flued heaters is greatly impaired due to flue installation restrictions.
In contrast, dedicated low emission gas heaters do not require a flue system. Furthermore, with the introduction of oxygen depletion sensors and thermostatic controls, they do not place critical reliance on ventilation as had been the case. These heaters can be positioned more conveniently and centralised to affect optimum warm air distribution. By definition unflued low NOx gas heaters are 100% efficient as all heat energy released from the flame is converted to useful heat.

Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining.

Nitrogen dioxide is a chemical compound with the formula NO2. One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, NO2 is an intermediate in the synthesis of nitric acid, millions of tons of which are produced each year, primarily for the production of fertilizers.

Chemiluminescence is the emission of light (luminescence) as the result of a chemical reaction. There may also be limited emission of heat. Given reactants A and B, with an excited intermediate ◊,
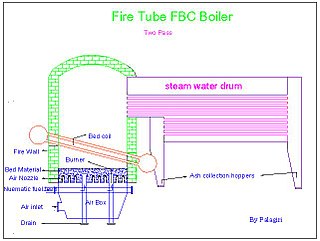
Fluidized bed combustion (FBC) is a combustion technology used to burn solid fuels.

Exhaust gas or flue gas is emitted as a result of the combustion of fuels such as natural gas, gasoline (petrol), diesel fuel, fuel oil, biodiesel blends, or coal. According to the type of engine, it is discharged into the atmosphere through an exhaust pipe, flue gas stack, or propelling nozzle. It often disperses downwind in a pattern called an exhaust plume.

A gas stove is a stove that is fuelled by combustible gas such as natural gas, propane, butane, liquefied petroleum gas, syngas, or other flammable gas. Before the advent of gas, cooking stoves relied on solid fuels such as coal or wood. The first gas stoves were developed in the 1820s and a gas stove factory was established in England in 1836. This new cooking technology had the advantage of being easily adjustable and could be turned off when not in use. The gas stove, however, did not become a commercial success until the 1880s, by which time supplies of piped gas were available in cities and large towns in Britain. The stoves became widespread on the European Continent and in the United States in the early 20th century.

A gas heater is a space heater used to heat a room or outdoor area by burning natural gas, liquefied petroleum gas, propane, or butane.

Flue gas is the gas exiting to the atmosphere via a flue, which is a pipe or channel for conveying exhaust gases, as from a fireplace, oven, furnace, boiler or steam generator. It often refers to the exhaust gas of combustion at power plants. Technology is available to remove pollutants from flue gas at power plants.
A nitrogen oxide sensor or NOx sensor is typically a high-temperature device built to detect nitrogen oxides in combustion environments such as an automobile, truck tailpipe or smokestack.

Coal pollution mitigation, sometimes labeled as clean coal, is a series of systems and technologies that seek to mitigate health and environmental impact of burning coal for energy. Burning coal releases harmful substances, including mercury, lead, sulfur dioxide (SO2), nitrogen oxides (NOx), and carbon dioxide (CO2), contributing to air pollution, acid rain, and greenhouse gas emissions. Methods include flue-gas desulfurization, selective catalytic reduction, electrostatic precipitators, and fly ash reduction focusing on reducing the emissions of these harmful substances. These measures aim to reduce coal's impact on human health and the environment.
In atmospheric chemistry, NOx is shorthand for nitric oxide and nitrogen dioxide, the nitrogen oxides that are most relevant for air pollution. These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone.

A kerosene heater, also known as a paraffin heater, is typically a portable, unvented, kerosene-fueled, space heating device. In Japan and other countries, they are a primary source of home heat. In the United States and Australia, they are a supplemental heat or a source of emergency heat during a power outage. Most kerosene heaters produce between 3.3 and 6.8 kilowatts.
The Keystone Generating Station is a 1.71-gigawatt, coal power plant located on roughly 1,500 acres (610 ha) in Plumcreek Township, southeastern Armstrong County, Pennsylvania near Crooked Creek, just west of Shelocta, Pennsylvania.

A flue-gas stack, also known as a smoke stack, chimney stack or simply as a stack, is a type of chimney, a vertical pipe, channel or similar structure through, which combustion product gases called flue gases are exhausted to the outside air. Flue gases are produced when coal, oil, natural gas, wood or any other fuel is combusted in an industrial furnace, a power plant's steam-generating boiler, or other large combustion device. Flue gas is usually composed of carbon dioxide (CO2) and water vapor, as well as nitrogen and excess oxygen remaining from the intake combustion air. It also contains a small percentage of pollutants such as particulate matter, carbon monoxide, nitrogen oxides and sulfur oxides. The flue gas stacks are often quite tall, up to 420 metres (1,380 ft), to increase the stack effect and dispersion of pollutants.

Air pollution is the contamination of air due to the presence of substances called pollutants in the atmosphere that are harmful to the health of humans and other living beings, or cause damage to the climate or to materials. It is also the contamination of the indoor or outdoor environment either by chemical, physical, or biological agents that alters the natural features of the atmosphere. There are many different types of air pollutants, such as gases, particulates, and biological molecules. Air pollution can cause diseases, allergies, and even death to humans; it can also cause harm to other living organisms such as animals and crops, and may damage the natural environment or built environment. Air pollution can be caused by both human activities and natural phenomena.

A wood-burning stove is a heating or cooking appliance capable of burning wood fuel, often called solid fuel, and wood-derived biomass fuel, such as sawdust bricks. Generally the appliance consists of a solid metal closed firebox, often lined by fire brick, and one or more air controls. The first wood-burning stove was patented in Strasbourg in 1557. This was two centuries before the Industrial Revolution, so iron was still prohibitively expensive. The first wood-burning stoves were high-end consumer items and only gradually became used widely.
Post-combustion capture refers to the removal of carbon dioxide (CO2) from a power station flue gas prior to its compression, transportation and storage in suitable geological formations, as part of carbon capture and storage. A number of different techniques are applicable, almost all of which are adaptations of acid gas removal processes used in the chemical and petrochemical industries. Many of these techniques existed before World War II and, consequently, post-combustion capture is the most developed of the various carbon-capture methodologies.
The SNOX process is a process which removes sulfur dioxide, nitrogen oxides and particulates from flue gases. The sulfur is recovered as concentrated sulfuric acid and the nitrogen oxides are reduced to free nitrogen. The process is based on the well-known wet sulfuric acid process (WSA), a process for recovering sulfur from various process gasses in the form of commercial quality sulfuric acid (H2SO4).
Computational fluid dynamics (CFD) are used to understand complex thermal flow regimes in power plants. The thermal power plant may be divided into different subsectors and the CFD analysis applied to critical equipment/components - mainly different types of heat exchangers - which are of crucial significance for efficient and trouble free long-term operation of the plant.

Nitrogen dioxide poisoning is the illness resulting from the toxic effect of nitrogen dioxide. It usually occurs after the inhalation of the gas beyond the threshold limit value. Nitrogen dioxide is reddish-brown with a very harsh smell at high concentrations, at lower concentrations it is colorless but may still have a harsh odour. Nitrogen dioxide poisoning depends on the duration, frequency, and intensity of exposure.