Related Research Articles

Anatomical pathology (Commonwealth) or anatomic pathology (U.S.) is a medical specialty that is concerned with the diagnosis of disease based on the macroscopic, microscopic, biochemical, immunologic and molecular examination of organs and tissues. Over the 20th century, surgical pathology has evolved tremendously: from historical examination of whole bodies (autopsy) to a more modernized practice, centered on the diagnosis and prognosis of cancer to guide treatment decision-making in oncology. Its modern founder was the Italian scientist Giovan Battista Morgagni from Forlì.

Clinical chemistry is a division in medical laboratory sciences focusing on qualitative tests of important compounds, referred to as analytes or markers, in bodily fluids and tissues using analytical techniques and specialized instruments. This interdisciplinary field includes knowledge from medicine, biology, chemistry, biomedical engineering, informatics, and an applied form of biochemistry.

Phlebotomy is the process of making a puncture in a vein, usually in the arm, with a cannula for the purpose of drawing blood. The procedure itself is known as a venipuncture, which is also used for intravenous therapy. A person who performs a phlebotomy is called a phlebotomist, although most doctors, nurses, and other technicians can also carry out a phlebotomy. In contrast, phlebectomy is the removal of a vein.

Pharmacy is the science and practice of discovering, producing, preparing, dispensing, reviewing and monitoring medications, aiming to ensure the safe, effective, and affordable use of medicines. It is a miscellaneous science as it links health sciences with pharmaceutical sciences and natural sciences. The professional practice is becoming more clinically oriented as most of the drugs are now manufactured by pharmaceutical industries. Based on the setting, pharmacy practice is either classified as community or institutional pharmacy. Providing direct patient care in the community of institutional pharmacies is considered clinical pharmacy.
Fisher Scientific International, Inc. was a laboratory supply and biotechnology company that provided products and services to the global scientific research and clinical laboratory markets until its merger with Thermo Electron in 2006, after which it became Thermo Fisher Scientific. The company offered products and services to over 350,000 customers located in approximately 150 countries including pharmaceutical and biotechnology companies, secondary and higher education institutions, hospitals and medical research institutions, and quality control, process control and research and development laboratories.
Transferrin saturation (TS), measured as a percentage, is a medical laboratory value. It is the value of serum iron divided by the total iron-binding capacity of the available transferrin, the main protein that binds iron in the blood, this value tells a clinician how much serum iron is bound. For instance, a value of 15% means that 15% of iron-binding sites of transferrin are being occupied by iron. The three results are usually reported together. A low transferrin saturation is a common indicator of iron deficiency anemia whereas a high transferrin saturation may indicate iron overload or hemochromatosis. Transferrin saturation is also called transferrin saturation index (TSI) or transferrin saturation percentage (TS%)

A medical laboratory scientist (MLS) or clinical laboratory scientist (CLS) or medical technologist (MT) performs diagnostic testing of blood and body fluids in clinical laboratories. The scope of a medical laboratory scientist's work begins with the receipt of patient or client specimens and terminates with the delivery of test results to physicians and other healthcare providers. The utility of clinical diagnostic testing relies squarely on the validity of test methodology. To this end, much of the work done by medical laboratory scientists involves ensuring specimen quality, interpreting test results, data-logging, testing control products, performing calibration, maintenance, validation, and troubleshooting of instrumentation as well as performing statistical analyses to verify the accuracy and repeatability of testing. Medical laboratory scientists may also assist healthcare providers with test selection and specimen collection and are responsible for prompt verbal delivery of critical lab results. Medical Laboratory Scientists in healthcare settings also play an important role in clinical diagnosis. An estimated 70% of medical decisions are based on laboratory test results and MLS contributions affect 95% of a health system's costs.
ISO 15189 Medical laboratories — Requirements for quality and competence is an international standard that specifies the quality management system requirements particular to medical laboratories. The standard was developed by the International Organisation for Standardization's Technical Committee 212. ISO/TC 212 assigned ISO 15189 to a working group to prepare the standard based on the details of ISO/IEC 17025:1999 General requirements for the competence of testing and calibration laboratories. This working group included provision of advice to medical laboratory users, including specifics on the collection of patient samples, the interpretation of test results, acceptable turnaround times, how testing is to be provided in a medical emergency, and the lab's role in the education and training of health care staff. While the standard is based on ISO/IEC 17025 and ISO 9001, it is a unique document that takes into consideration the specific requirements of the medical environment and the importance of the medical laboratory to patient care.

The Health Sciences Authority (HSA) is a statutory board under the Ministry of Health of the Government of Singapore. It is a multi-disciplinary agency responsible for applying medical, pharmaceutical, and scientific expertise to protect and advance public health and safety.
The International Federation of Clinical Chemistry and Laboratory Medicine or IFCC is a global organization that promotes the fields of clinical chemistry and laboratory medicine. It was established in 1952 as the International Association of Clinical Biochemists to organize the various national societies of these fields. The organization aims to transcend the boundaries of the field of clinical chemistry and laboratory medicine, to build professionalism of members worldwide, to disseminate information on ”best practice” at various levels of technology and of economic development, to provide a forum of standardization and traceability, to enhance the scientific level and the quality of diagnosis and therapy for patients.
UpToDate, Inc. is a company in the Wolters Kluwer Health division of Wolters Kluwer, the main product of which is the eponymous UpToDate, a software system that is a point-of-care medical resource.
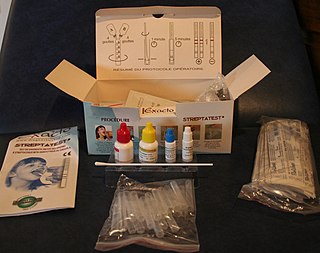
Point-of-care testing (POCT), also called near-patient testing or bedside testing, is defined as medical diagnostic testing at or near the point of care—that is, at the time and place of patient care. This contrasts with the historical pattern in which testing was wholly or mostly confined to the medical laboratory, which entailed sending off specimens away from the point of care and then waiting hours or days to learn the results, during which time care must continue without the desired information.
ConsumerLab.com, LLC. is a privately held American company registered in White Plains, NY. It is a publisher of test results on health, wellness, and nutrition products. Consumer Labs is not a laboratory, but contracts studies to outside testing laboratories. It purchases dietary supplement products and other consumer goods directly from public storefronts and online retailers, contracts for testing by private laboratories, and publishes reports based on the results. It primarily derives revenue from the sale of subscriptions to its online publications, which are paywalled. Other sources of revenue include a proprietary certification program, licensing fees, contents re-publication license fees, and advertising.

A medical laboratory or clinical laboratory is a laboratory where tests are conducted out on clinical specimens to obtain information about the health of a patient to aid in diagnosis, treatment, and prevention of disease. Clinical medical laboratories are an example of applied science, as opposed to research laboratories that focus on basic science, such as found in some academic institutions.
Laboratory Corporation of America Holdings, more commonly known as Labcorp, is an American healthcare company headquartered in Burlington, North Carolina. It operates one of the largest clinical laboratory networks in the world, with a United States network of 36 primary laboratories. Before a merger with National Health Laboratory in 1995, the company operated under the name Roche BioMedical. Labcorp performs its largest volume of specialty testing at its Center for Esoteric Testing in Burlington, North Carolina, where the company is headquartered. As of 2018, Labcorp processes 2.5 million lab tests weekly.
The Association for Diagnostics & Laboratory Medicine is a global scientific society dedicated to clinical laboratory science and its application to healthcare. ADLM's current president is Octavia M. Peck Palmer, PhD, FAAC, and the association headquarters are located in Washington, D.C..
The American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) is a medical society for the medical subspecialty of neuromuscular and electrodiagnostic medicine based in the United States. Members are primarily neurologists and physiatrists—as well as allied health professionals and PhD researchers.
The European Federation of Clinical Chemistry and Laboratory Medicine (EFLM)(formerly EFCC) is a federation of national member societies of clinical chemistry and laboratory medicine from Europe. EFLM has its registered office in Brussels and administrative office in Milan. EFLM is the European Region member of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)
The Association for Molecular Pathology is a professional association of individuals serving patients through molecular diagnostics testing. Founded in 1995, the Association has more than 2,800 members in over 50 countries.
LigoLab Information System is an American software company that provides software and laboratory operating systems for clinical laboratories. LigoLab develops and distributes the software tool TestDirectly, which is used for COVID-19 testing. It is based in Glendale, California.
References
- 1 2 Campbell, B; Linzer, G; Dufour, DR (May 2014). "Lab Tests Online and consumer understanding of laboratory testing". Clin Chim Acta. 432: 162–5. doi:10.1016/j.cca.2013.09.028. PMID 24121032.
- 1 2 Linzer, George (July 5, 2011). "Lab Tests Online at 10 Years: 100 Million Served". Clinical Lab Products. Clinical Lab Products. Clinical Lab Products.
- ↑ Kulkarni, Shweta (13 July 2012). "Lab Tests Online Honoured With 2012 Communicator Award". Medtech Europe. Archived from the original on 8 February 2015. Retrieved 8 February 2015.
- ↑ An Overview of Lab Tests Online (PDF). Clinical Laboratory Improvement Advisory Committee. p. 6. Archived from the original (PDF) on 2017-02-22. Retrieved 8 February 2015.
- ↑ Chisolm, Stephanie (Jul 3, 2008). The Health Professions: Trends and Opportunities in U.S. Health Care. Jones & Bartlett Publishers. p. 116. ISBN 978-0763735203.
- ↑ "Lab Tests Online". American Association for Clinical Chemistry. Retrieved 17 October 2017.
- ↑ "About Lab Tests Online | Lab Tests Online". labtestsonline.org. Retrieved 2021-05-14.
- ↑ "LabTestsOnline.org is now Testing.com". Testing.com. 2021-11-08. Retrieved 2021-11-15.