Related Research Articles

Gram-negative bacteria are bacteria that unlike gram-positive bacteria do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. Their defining characteristic is their cell envelope, which consists of a thin peptidoglycan cell wall sandwiched between an inner (cytoplasmic) membrane and an outer membrane. These bacteria are found in all environments that support life on Earth.
Peptidoglycan or murein is a unique large macromolecule, a polysaccharide, consisting of sugars and amino acids that forms a mesh-like layer (sacculus) that surrounds the bacterial cytoplasmic membrane. The sugar component consists of alternating residues of β-(1,4) linked N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM). Attached to the N-acetylmuramic acid is an oligopeptide chain made of three to five amino acids. The peptide chain can be cross-linked to the peptide chain of another strand forming the 3D mesh-like layer. Peptidoglycan serves a structural role in the bacterial cell wall, giving structural strength, as well as counteracting the osmotic pressure of the cytoplasm. This repetitive linking results in a dense peptidoglycan layer which is critical for maintaining cell form and withstanding high osmotic pressures, and it is regularly replaced by peptidoglycan production. Peptidoglycan hydrolysis and synthesis are two processes that must occur in order for cells to grow and multiply, a technique carried out in three stages: clipping of current material, insertion of new material, and re-crosslinking of existing material to new material.

Bacteriocins are proteinaceous or peptidic toxins produced by bacteria to inhibit the growth of similar or closely related bacterial strain(s). They are similar to yeast and paramecium killing factors, and are structurally, functionally, and ecologically diverse. Applications of bacteriocins are being tested to assess their application as narrow-spectrum antibiotics.

Lanthionine is a nonproteinogenic amino acid with the chemical formula (HOOC-CH(NH2)-CH2-S-CH2-CH(NH2)-COOH). It is typically formed by a cysteine residue and a dehydrated serine residue. Despite its name, lanthionine does not contain the element lanthanum.
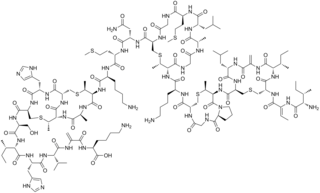
Nisin is a polycyclic antibacterial peptide produced by the bacterium Lactococcus lactis that is used as a food preservative. It has 34 amino acid residues, including the uncommon amino acids lanthionine (Lan), methyllanthionine (MeLan), didehydroalanine (Dha), and didehydroaminobutyric acid (Dhb). These unusual amino acids are introduced by posttranslational modification of the precursor peptide. In these reactions a ribosomally synthesized 57-mer is converted to the final peptide. The unsaturated amino acids originate from serine and threonine, and the enzyme-catalysed addition of cysteine residues to the didehydro amino acids result in the multiple (5) thioether bridges.

Polymyxins are antibiotics. Polymyxins B and E are used in the treatment of Gram-negative bacterial infections. They work mostly by breaking up the bacterial cell membrane. They are part of a broader class of molecules called nonribosomal peptides.

Gramicidin, also called gramicidin D, is a mix of ionophoric antibiotics, gramicidin A, B and C, which make up about 80%, 5%, and 15% of the mix, respectively. Each has 2 isoforms, so the mix has 6 different types of gramicidin molecules. They can be extracted from Brevibacillus brevis soil bacteria. Gramicidins are linear peptides with 15 amino acids. This is in contrast to unrelated gramicidin S, which is a cyclic peptide.

Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antimicrobials which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators.
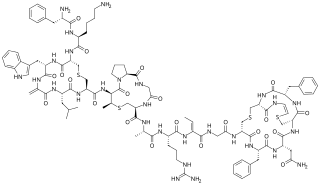
Mutacin 1140 is a bacteriocin produced by Streptococcus mutans. It has activity against a broad spectrum of Gram-positive bacteria. It is a member of the class of compounds known as lantibiotics.

Tyrocidine is a mixture of cyclic decapeptides produced by the bacteria Brevibacillus brevis found in soil. It can be composed of 4 different amino acid sequences, giving tyrocidine A–D. Tyrocidine is the major constituent of tyrothricin, which also contains gramicidin. Tyrocidine was the first commercially available antibiotic, but has been found to be toxic toward human blood and reproductive cells. The function of tyrocidine within its host B. brevis is thought to be regulation of sporulation.
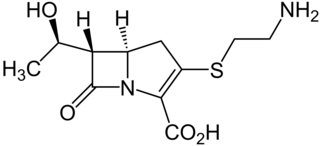
Thienamycin is one of the most potent naturally produced antibiotics known thus far, discovered in Streptomyces cattleya in 1976. Thienamycin has excellent activity against both Gram-positive and Gram-negative bacteria and is resistant to bacterial β-lactamase enzymes. Thienamycin is a zwitterion at pH 7.

Class II bacteriocins are a class of small peptides that inhibit the growth of various bacteria.

Thiostrepton is a natural cyclic oligopeptide antibiotic of the thiopeptide class, derived from several strains of streptomycetes, such as Streptomyces azureus and Streptomyces laurentii. Thiostrepton is a natural product of the ribosomally synthesized and post-translationally modified peptide (RiPP) class.

Ramoplanin (INN) is a glycolipodepsipeptide antibiotic drug derived from strain ATCC 33076 of Actinoplanes. It is effective against Gram-positive bacteria.
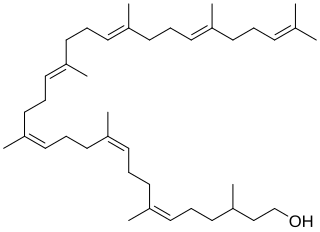
Bactoprenol also known as dolichol-11 and C55-isoprenyl alcohol (C55-OH) is a lipid first identified in certain species of lactobacilli. It is a hydrophobic alcohol that plays a key role in the growth of cell walls (peptidoglycan) in Gram-positive bacteria.
Ribosomally synthesized and post-translationally modified peptides (RiPPs), also known as ribosomal natural products, are a diverse class of natural products of ribosomal origin. Consisting of more than 20 sub-classes, RiPPs are produced by a variety of organisms, including prokaryotes, eukaryotes, and archaea, and they possess a wide range of biological functions.

Lipid II is a precursor molecule in the synthesis of the cell wall of bacteria. It is a peptidoglycan, which is amphipathic and named for its bactoprenol hydrocarbon chain, which acts as a lipid anchor, embedding itself in the bacterial cell membrane. Lipid II must translocate across the cell membrane to deliver and incorporate its disaccharide-pentapeptide "building block" into the peptidoglycan mesh. Lipid II is the target of several antibiotics.
Copsin is a fungal defensin that acts as an antimicrobial polypeptide secreted from the inky cap mushroom, first reported at the end of 2014. The fungal defensin acts against gram positive bacteria.
Heike Brötz-Oesterhelt is a German microbiologist. She is a full professor and holds the Chair of the Department for Microbial Bioactive Compounds at the Interfaculty Institute for Microbiology and Infection Medicine, University of Tübingen, Germany.

Cinnamycin is a tetracyclic antibacterial peptide produced by Streptomyces cinnamoneus containing 19 amino acid residues including the unusual amino acids threo-3-methyl-lanthionine, meso-lanthionine, lysinoalanine, and 3-hydroxyaspartic acid.
References
- ↑ Chatterjee C, Paul M, Xie L, van der Donk WA (February 2005). "Biosynthesis and mode of action of lantibiotics". Chem. Rev. 105 (2): 633–84. doi:10.1021/cr030105v. PMID 15700960.
- 1 2 Kellner R, Jung G, Hörner T, Zähner H, Schnell N, Entian KD, Götz F (October 1988). "Gallidermin: a new lanthionine-containing polypeptide antibiotic". Eur. J. Biochem. 177 (1): 53–9. doi:10.1111/j.1432-1033.1988.tb14344.x. PMID 3181159.
- 1 2 Sass P, Jansen A, Szekat C, Sass V, Sahl HG, Bierbaum G (2008). "The lantibiotic mersacidin is a strong inducer of the cell wall stress response of Staphylococcus aureus". BMC Microbiol. 8: 186. doi: 10.1186/1471-2180-8-186 . PMC 2592248 . PMID 18947397.
- ↑ Brötz H, Bierbaum G, Markus A, Molitor E, Sahl HG (March 1995). "Mode of action of the lantibiotic mersacidin: inhibition of peptidoglycan biosynthesis via a novel mechanism?". Antimicrob. Agents Chemother. 39 (3): 714–9. doi:10.1128/AAC.39.3.714. PMC 162610 . PMID 7793878.
- 1 2 Makino A, Baba T, Fujimoto K, Iwamoto K, Yano Y, Terada N, Ohno S, Sato SB, Ohta A, Umeda M, Matsuzaki K, Kobayashi T (January 2003). "Cinnamycin (Ro 09-0198) promotes cell binding and toxicity by inducing transbilayer lipid movement". J. Biol. Chem. 278 (5): 3204–9. doi: 10.1074/jbc.M210347200 . PMID 12446685.
- ↑ Cooper LE, McClerren AL, Chary A, van der Donk WA (October 2008). "Structure-activity relationship studies of the two-component lantibiotic haloduracin". Chem. Biol. 15 (10): 1035–45. doi:10.1016/j.chembiol.2008.07.020. PMC 2633096 . PMID 18940665.
- ↑ Stein T (May 2005). "Bacillus subtilis antibiotics: structures, syntheses and specific functions". Mol. Microbiol. 56 (4): 845–57. doi: 10.1111/j.1365-2958.2005.04587.x . PMID 15853875. S2CID 20144405.
- ↑ Oman TJ, Boettcher JM, Wang H, Okalibe XN, van der Donk WA (February 2011). "Sublancin is not a lantibiotic but an S-linked glycopeptide". Nat. Chem. Biol. 7 (2): 78–80. doi:10.1038/nchembio.509. PMC 3060661 . PMID 21196935.
- ↑ Siegers K, Heinzmann S, Entian KD (May 1996). "Biosynthesis of lantibiotic nisin. Posttranslational modification of its prepeptide occurs at a multimeric membrane-associated lanthionine synthetase complex". J. Biol. Chem. 271 (21): 12294–301. doi: 10.1074/jbc.271.21.12294 . PMID 8647829.
- ↑ Goto, Y; Li, B; Claesen, J; Shi, Y; Bibb, MJ; van der Donk, WA (2010). "Discovery of unique lanthionine synthetases reveals new mechanistic and evolutionary insights". PLOS Biology. 8 (3): e1000339. doi: 10.1371/journal.pbio.1000339 . PMC 2843593 . PMID 20351769.
- ↑ Zhang, Q; Yu, Y; Vélasquez, JE; van der Donk, WA (2012). "Evolution of lanthipeptide synthetases". Proceedings of the National Academy of Sciences. 109 (45): 18361–6. Bibcode:2012PNAS..10918361Z. doi: 10.1073/pnas.1210393109 . PMC 3494888 . PMID 23071302.
- ↑ Brötz H, Sahl HG (2000). "New insights into the mechanism of action of lantibiotics—diverse biological effects by binding to the same molecular target". Journal of Antimicrobial Chemotherapy . 46 (1): 1–6. doi: 10.1093/jac/46.1.1 . PMID 10882681.
- ↑ Cotter, Hill, Ross (2005). "Bacterial Lantibiotics: Strategies to Improve Therapeutic Potential" (PDF). Current Protein & Peptide Science. 6 (1): 61–75. doi:10.2174/1389203053027584. PMID 15638769. Archived from the original (PDF) on 2007-09-28. Retrieved 2007-06-01.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - 1 2 van Kraaij C, de Vos WM, Siezen RJ, Kuipers OP (October 1999). "Lantibiotics: biosynthesis, mode of action and applications". Nat Prod Rep. 16 (5): 575–87. CiteSeerX 10.1.1.546.6212 . doi:10.1039/a804531c. PMID 10584332.
- ↑ "New antibiotic compound enters phase I clinical trial". Press Release. Wellcome Trust. 2011-11-03.
- ↑ Parker S (2012-08-06). "Novacta Biosystems Limited completes Phase I study of NVB302 against C. difficile infection in healthy volunteers". Press Release. Celtic Pharma Holding. Archived from the original on 2013-09-01. Retrieved 2013-03-23.
- ↑ Hammami R, Zouhir A, Ben Hamida J, Fliss I (2007). "BACTIBASE: a new web-accessible database for bacteriocin characterization". BMC Microbiology. 7: 89. doi: 10.1186/1471-2180-7-89 . PMC 2211298 . PMID 17941971.
- ↑ Hammami R, Zouhir A, Le Lay C, Ben Hamida J, Fliss I (2010). "BACTIBASE second release: a database and tool platform for bacteriocin characterization". BMC Microbiology. 10: 22. doi: 10.1186/1471-2180-10-22 . PMC 2824694 . PMID 20105292.
- ↑ "DBT Centre for Bioinformatics Presidency University, Kolkata". Archived from the original on 2013-08-15. Retrieved 2013-07-25.