Related Research Articles
Ferroelectricity is a characteristic of certain materials that have a spontaneous electric polarization that can be reversed by the application of an external electric field. All ferroelectrics are pyroelectric, with the additional property that their natural electrical polarization is reversible. The term is used in analogy to ferromagnetism, in which a material exhibits a permanent magnetic moment. Ferromagnetism was already known when ferroelectricity was discovered in 1920 in Rochelle salt by Joseph Valasek. Thus, the prefix ferro, meaning iron, was used to describe the property despite the fact that most ferroelectric materials do not contain iron. Materials that are both ferroelectric and ferromagnetic are known as multiferroics.
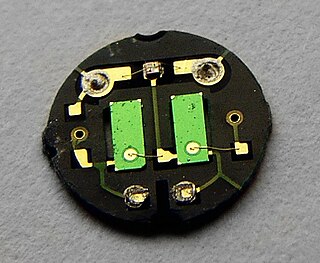
Pyroelectricity is a property of certain crystals which are naturally electrically polarized and as a result contain large electric fields. Pyroelectricity can be described as the ability of certain materials to generate a temporary voltage when they are heated or cooled. The change in temperature modifies the positions of the atoms slightly within the crystal structure, such that the polarization of the material changes. This polarization change gives rise to a voltage across the crystal. If the temperature stays constant at its new value, the pyroelectric voltage gradually disappears due to leakage current. The leakage can be due to electrons moving through the crystal, ions moving through the air, or current leaking through a voltmeter attached across the crystal.

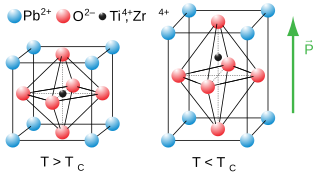
A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two ions, often of very different sizes, and X is an ion (frequently oxide) that bonds to both ions. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in their oxidation states.

A thermographic camera is a device that creates an image using infrared (IR) radiation, similar to a normal camera that forms an image using visible light. Instead of the 400–700 nanometre (nm) range of the visible light camera, infrared cameras are sensitive to wavelengths from about 1,000 nm to about 14,000 nm (14 μm). The practice of capturing and analyzing the data they provide is called thermography.

Strontium titanate is an oxide of strontium and titanium with the chemical formula SrTiO3. At room temperature, it is a centrosymmetric paraelectric material with a perovskite structure. At low temperatures it approaches a ferroelectric phase transition with a very large dielectric constant ~104 but remains paraelectric down to the lowest temperatures measured as a result of quantum fluctuations, making it a quantum paraelectric. It was long thought to be a wholly artificial material, until 1982 when its natural counterpart—discovered in Siberia and named tausonite—was recognised by the IMA. Tausonite remains an extremely rare mineral in nature, occurring as very tiny crystals. Its most important application has been in its synthesized form wherein it is occasionally encountered as a diamond simulant, in precision optics, in varistors, and in advanced ceramics.
Lead zirconate titanate is an inorganic compound with the chemical formula Pb[ZrxTi1-x]O3 (0≤x≤1). Also called lead zirconium titanate, it is a ceramic perovskite material that shows a marked piezoelectric effect, meaning that the compound changes shape when an electric field is applied. It is used in a number of practical applications such as ultrasonic transducers and piezoelectric resonators. It is a white to off-white solid.

Lithium tantalate (LiTaO3) is a perovskite which possesses unique optical, piezoelectric and pyroelectric properties which make it valuable for nonlinear optics, passive infrared sensors such as motion detectors, terahertz generation and detection, surface acoustic wave applications, cell phones and possibly pyroelectric nuclear fusion. Considerable information is available from commercial sources about this salt.
Electroceramics is a class of ceramic materials used primarily for their electrical properties.

Barium titanate (BTO) is an inorganic compound with chemical formula BaTiO3. Barium titanate appears white as a powder and is transparent when prepared as large crystals. It is a ferroelectric ceramic material that exhibits the photorefractive effect and piezoelectric properties. It is used in capacitors, electromechanical transducers and nonlinear optics.

Scandium(III) fluoride, ScF3, is an ionic compound. This salt is slightly soluble in water but dissolves in the presence of excess fluoride to form the ScF63− anion.
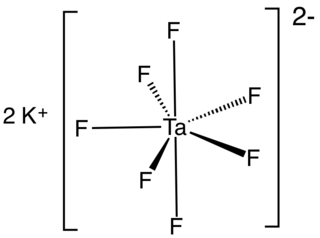
Tantalate is an tantalum-containing anion or a salt of such an anion. A commercially important example is heptafluorotantalate (TaF72−) and its potassium salt (K2TaF7).

Lead(II) titanate is an inorganic compound with the chemical formula PbTiO3. It is the lead salt of titanic acid. Lead(II) titanate is a yellow powder that is insoluble in water.
Europium barium titanate is a chemical compound composed of barium, europium, titanium, and oxygen. It is magnetic and ferroelectric at i.5 K.
LSAT is the most common name for the inorganic compound lanthanum aluminate - strontium aluminium tantalate, which has the chemical formula (LaAlO3)0.3(Sr2TaAlO6)0.7 or its less common alternative: (La0.18Sr0.82)(Al0.59Ta0.41)O3. LSAT is a hard, optically transparent oxide of the elements lanthanum, aluminum, strontium and tantalum. LSAT has the perovskite crystal structure, and its most common use is as a single crystal substrate for the growth of epitaxial thin films.
Relaxor ferroelectrics are ferroelectric materials that exhibit high electrostriction. As of 2015, although they have been studied for over fifty years, the mechanism for this effect is still not completely understood, and is the subject of continuing research.
A complex oxide is a chemical compound that contains oxygen and at least two other elements. Complex oxide materials are notable for their wide range of magnetic and electronic properties, such as ferromagnetism, ferroelectricity, and high-temperature superconductivity. These properties often come from their strongly correlated electrons in d or f orbitals.
Sodium bismuth titanate or bismuth sodium titanium oxide (NBT or BNT) is a solid inorganic compound of sodium, bismuth, titanium and oxygen with the chemical formula of Na0.5Bi0.5TiO3 or Bi0.5Na0.5TiO3. This compound adopts the perovskite structure.

Kenji Uchino is an American electronics engineer, physicist, academic, inventor and industry executive. He is currently a Professor of Electrical Engineering at Pennsylvania State University, where he also directs the International Center for Actuators and Transducers at Materials Research Institute. He is the former Associate Director at The US Office of Naval Research – Global Tokyo Office.
Nickel niobate is a complex oxide which as a solid material has found potential applications in catalysis and lithium batteries.
References
- ↑ Chu, Fan; Fox, Glen R.; Setter, Nava (21 January 2005). "Dielectric Properties of Complex Perovskite Lead Scandium Tantalate under dc Bias". Journal of the American Ceramic Society. 81 (6): 1577–1582. doi:10.1111/j.1151-2916.1998.tb02519.x.
{{cite journal}}:|access-date=requires|url=(help) - 1 2 Groves, P (10 December 1985). "Low-temperature studies of ferroelectric lead scandium tantalate". Journal of Physics C: Solid State Physics. 18 (34): L1073–L1078. doi:10.1088/0022-3719/18/34/002.
{{cite journal}}:|access-date=requires|url=(help) - ↑ De Kroon, A. P.; Dunn, S. C.; Whatmore, R. W. (February 2001). "Piezo- and pyroelectric properties of lead scandium tantalate thin films". Integrated Ferroelectrics. 35 (1–4): 209–218. doi:10.1080/10584580108016902.
{{cite journal}}:|access-date=requires|url=(help) - ↑ Todd, Michael A.; Donohue, Paul P.; Watton, Rex; Williams, Dennis J.; Anthony, Carl J.; Blamire, Mark G. (1 December 2002). "High-performance ferroelectric and magnetoresistive materials for next-generation thermal detector arrays": 88. doi:10.1117/12.452244.
{{cite journal}}: Cite journal requires|journal=(help)