Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently, its IUPAC systematic name is 2-aminopropanoic acid, and it is classified as a nonpolar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesised metabolically and does not need to be present in the diet. It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG).

Aspartic acid, is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of proteins. D-aspartic acid is one of two D-amino acids commonly found in mammals. Apart from a few rare exceptions, D-aspartic acid is not used for protein synthesis but is incorporated into some peptides and plays a role as a neurotransmitter/neuromodulator.

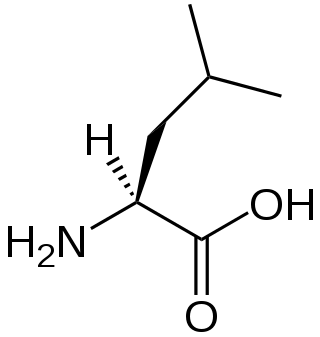
Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a hydrocarbon side chain with a branch (a central carbon atom bound to three other carbon atoms). It is classified as a non-polar, uncharged (at physiological pH), branched-chain, aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it. Essential amino acids are necessary in the human diet. In plants isoleucine can be synthesized from threonine and methionine. In plants and bacteria, isoleucine is synthesized from pyruvate employing leucine biosynthesis enzymes. It is encoded by the codons AUU, AUC, and AUA.

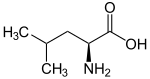
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isobutyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, and beans and other legumes. It is encoded by the codons UUA, UUG, CUU, CUC, CUA, and CUG.

Flavins refers generally to the class of organic compounds containing the tricyclic heterocycle isoalloxazine or its isomer alloxazine, and derivatives thereof. The biochemical source of flavin is the yellow B vitamin riboflavin. The flavin moiety is often attached with an adenosine diphosphate to form flavin adenine dinucleotide (FAD), and, in other circumstances, is found as flavin mononucleotide, a phosphorylated form of riboflavin. It is in one or the other of these forms that flavin is present as a prosthetic group in flavoproteins. Despite the similar names, flavins are chemically and biologically distinct from the flavanoids, and the flavonols.

Anabolism is the set of metabolic pathways that construct molecules from smaller units. These reactions require energy, known also as an endergonic process. Anabolism is the building-up aspect of metabolism, whereas catabolism is the breaking-down aspect. Anabolism is usually synonymous with biosynthesis.

Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino acids, 20 in the standard genetic code and an additional 2 that can be incorporated by special translation mechanisms.

In chemistry, a molecule or ion is called chiral if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality. The terms are derived from Ancient Greek χείρ (cheir) 'hand'; which is the canonical example of an object with this property.

Maple syrup urine disease (MSUD) is an autosomal recessive metabolic disorder affecting branched-chain amino acids. It is one type of organic acidemia. The condition gets its name from the distinctive sweet odor of affected infants' urine and earwax, particularly prior to diagnosis and during times of acute illness. It was described by John Menkes in the 1950s.
A branched-chain amino acid (BCAA) is an amino acid having an aliphatic side-chain with a branch. Among the proteinogenic amino acids, there are three BCAAs: leucine, isoleucine, and valine. Non-proteinogenic BCAAs include 2-aminoisobutyric acid and alloisoleucine.

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).

The acetolactate synthase (ALS) enzyme is a protein found in plants and micro-organisms. ALS catalyzes the first step in the synthesis of the branched-chain amino acids.

A ketogenic amino acid is an amino acid that can be degraded directly into acetyl-CoA, which is the precursor of ketone bodies and myelin, particularly during early childhood, when the developing brain requires high rates of myelin synthesis. This is in contrast to the glucogenic amino acids, which are converted into glucose. Ketogenic amino acids are unable to be converted to glucose as both carbon atoms in the ketone body are ultimately degraded to carbon dioxide in the citric acid cycle.
In enzymology, an isoleucine—tRNA ligase is an enzyme that catalyzes the chemical reaction
In enzymology, a leucine—tRNA ligase is an enzyme that catalyzes the chemical reaction

Ergocryptine is an ergopeptine and one of the ergoline alkaloids. It is isolated from ergot or fermentation broth and it serves as starting material for the production of bromocriptine. Two isomers of ergocryptine exist, α-ergocryptine and β-ergocryptine. The beta differs from the alpha form only in the position of a single methyl group, which is a consequence of the biosynthesis in which the proteinogenic amino acid leucine is replaced by isoleucine. β-Ergocryptine was first identified in 1967 by Albert Hofmann. Ergot from different sources have different ratios of the two isomers.

Norleucine (abbreviated as Nle) is an amino acid with the formula CH3(CH2)3CH(NH2)CO2H. A systematic name for this compound is 2-aminohexanoic acid. The compound is an isomer of the more common amino acid leucine. Like most other α-amino acids, norleucine is chiral. It is a white, water-soluble solid.

In biochemistry, non-coded or non-proteinogenic amino acids are distinct from the 22 proteinogenic amino acids which are naturally encoded in the genome of organisms for the assembly of proteins. However, over 140 non-proteinogenic amino acids occur naturally in proteins and thousands more may occur in nature or be synthesized in the laboratory. Chemically synthesized amino acids can be called unnatural amino acids. Unnatural amino acids can be synthetically prepared from their native analogs via modifications such as amine alkylation, side chain substitution, structural bond extension cyclization, and isosteric replacements within the amino acid backbone. Many non-proteinogenic amino acids are important:

l-Photo-leucine is a synthetic derivative of the l-leucine amino acid that is used as its natural analog and is characterized for having photo-reactivity, which makes it suitable for observing and characterizing protein-protein interactions (PPI). When a protein containing this amino acid (A) is exposed to ultraviolet light while interacting with another protein (B), the complex formed from these two proteins (AB) remains attached and can be isolated for study.
In chemical nomenclature, a descriptor is a notational prefix placed before the systematic substance name, which describes the configuration or the stereochemistry of the molecule. Some listed descriptors are only of historical interest and should not be used in publications anymore as they do not correspond with the modern recommendations of the IUPAC. Stereodescriptors are often used in combination with locants to clearly identify a chemical structure unambiguously.