
Plasmodium is a genus of unicellular eukaryotes that are obligate parasites of vertebrates and insects. The life cycles of Plasmodium species involve development in a blood-feeding insect host which then injects parasites into a vertebrate host during a blood meal. Parasites grow within a vertebrate body tissue before entering the bloodstream to infect red blood cells. The ensuing destruction of host red blood cells can result in malaria. During this infection, some parasites are picked up by a blood-feeding insect, continuing the life cycle.

Isosporiasis, also known as cystoisosporiasis, is a human intestinal disease caused by the parasite Cystoisospora belli. It is found worldwide, especially in tropical and subtropical areas. Infection often occurs in immuno-compromised individuals, notably AIDS patients, and outbreaks have been reported in institutionalized groups in the United States. The first documented case was in 1915. It is usually spread indirectly, normally through contaminated food or water (CDC.gov).

Eimeria is a genus of apicomplexan parasites that includes various species capable of causing the disease coccidiosis in animals such as cattle, poultry and smaller ruminants including sheep and goats. Eimeria species are considered to be monoxenous because the life cycle is completed within a single host, and stenoxenous because they tend to be host specific, although a number of exceptions have been identified. Species of this genus infect a wide variety of hosts. Thirty-one species are known to occur in bats (Chiroptera), two in turtles, and 130 named species infect fish. Two species infect seals. Five species infect llamas and alpacas: E. alpacae, E. ivitaensis, E. lamae, E. macusaniensis, and E. punonensis. A number of species infect rodents, including E. couesii, E. kinsellai, E. palustris, E. ojastii and E. oryzomysi. Others infect poultry, rabbits and cattle. For full species list, see below.

Babesia, also called Nuttallia, is an apicomplexan parasite that infects red blood cells and is transmitted by ticks. Originally discovered by the Romanian bacteriologist Victor Babeș in 1888, over 100 species of Babesia have since been identified.
Leucocytozoon andrewsi is a parasite of the genus Leucocytozoon.
Megaloschizonts are large schizonts that produce extremely high numbers of merozoites. They are found in various species of the Phylum Apicomplexa. The Apicomplexa phylum contains several parasitic protozoans. They have a very complex life cycle that includes several stages. Megaloschizonts and the smaller schizonts are the part of the life cycle that takes place inside the infected host organism and operates as an asexually reproductive cell. Megaloschizonts appear as grey-white nodules found in the smooth muscle of major organs, such as the heart, liver, lung or spleen.
Plasmodium uluguruense is a parasite of the genus Plasmodium subgenus Lacertamoeba.
Plasmodium loveridgei is a parasite of the genus Plasmodium subgenus Lacertamoeba.

Haemoproteus is a genus of alveolates that are parasitic in birds, reptiles and amphibians. Its name is derived from Greek: Haima, "blood", and Proteus, a sea god who had the power of assuming different shapes. The name Haemoproteus was first used in the description of H. columbae in the blood of the pigeon Columba livia by Kruse in 1890. This was also the first description of this genus. Two other genera — Halteridium and Simondia — are now considered to be synonyms of Haemoproteus.
Plasmodium juxtanucleare is a species of parasite in the family Plasmodiidae. The vertebrate hosts for this parasite are birds.

Adeleorina is a suborder of parasites in the phylum Apicomplexa.
Karyolysus is a genus of coccidia. With the exception of K. sonomae whose vertebrate host is the yellow-legged frog, species in this genus only infect lizards of the genus Lacerta.
Plasmodium vaughani is a parasite of the genus Plasmodium, and the type species of the subgenus Novyella. As in all Plasmodium species, P. vaughani has both vertebrate and insect hosts. The vertebrate hosts for this parasite are birds.
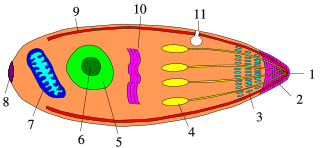
Apicomplexans, a group of intracellular parasites, have life cycle stages that allow them to survive the wide variety of environments they are exposed to during their complex life cycle. Each stage in the life cycle of an apicomplexan organism is typified by a cellular variety with a distinct morphology and biochemistry.
Dactylosoma is a genus of parasitic alveolates of the phylum Apicomplexa.
Leucocytozoon caulleryi is a species of the genus Leucocytozoon, a genus of parasitic alveolates.
The genus Schellackia comprises obligate unicellular eukaryotic parasites within the phylum Apicomplexa, and infects numerous species of lizards and amphibians worldwide. Schellackia is transmitted via insect vectors, primarily mites and mosquitoes, which take up the parasite in blood meals. These vectors then subsequently infect reptilian and amphibian which consume the infected insects. The parasites deform erythrocytes of the host into crescents, and can be visualized using a blood smear.
Atoxoplasma is a genus of parasitic alveolates in the phylum Apicomplexa. The species in this genus infect birds. They are spread by the orofaecal route.
Nephroisospora is a genus of parasites that infects bats
The Ophryocystidae are a family of parasites in the phylum Apicomplexa. Species in this family infect insects.









