List
| Precursor, name, formula | CAS No. | Chemical stability | Themal stability | Evaporation T (pressure) | Vapour pressure (oC/Torr) | Decomposition T | Oligommerization | Crystal structure | Melting point | TG data | DSC | IR spectra | NMR data | Solubility | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li(TMHD), Lithium tetramethylheptanedionate, C11H19LiO2 | 22441-13-0 | Decomposes at low pressure and room temperatures, [1] stable under N2 or Ar in sealed contanier and decomposes slowly in contact with moist air and rapidly in contact with water. | Above 215 °C under high vacuum it decomposes to form ketenes and carbanions [1] | 268-270 °C (atmospehric pressure) | NA | 265-268 °C | Soluble in water | [1] D. Saulys, V. Joshkin, M. Khoudiakov, T.F. Kuech, A.B. Ellis, S.R. Oktyabrsky, L. McCaughan, Journal of Crystal Growth 217 (2000) 287-301 | |||||||
| Lithium bis(trimethylsilyl)amide, LiN(SiMe3)2 | 4039- | Reacts violently with water. | 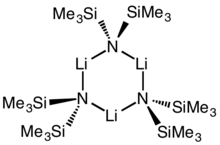 | 70-72 °C | J. Hamalainen, J. Holopainen, F. Munnik, T. Hatanpaa, M. Heikkila, M. Ritala, and M. Leskela, J Electrochem Soc, 159, A259 (2012). | ||||||||||
| Lithium bis(ethyldimethylsilyl)amide, [Li(NSiMe2Et)2]2 | 300585-49-3 | 122/0.2 | Broomhall-Dillard, R. N. R., Gordon, R. G., & Wagner, V. A., MRS Proceedings, 1999, 606 | ||||||||||||
| Lithium tert-amyl(i-propyldimethylsilyl)amide | 137/0.2 | Broomhall-Dillard, R. N. R., Gordon, R. G., & Wagner, V. A., MRS Proceedings, 1999, 606 | |||||||||||||
| Lithium bis(3,3-dimethylbutyldimethylsilyl)amide | 225/0.9 | Broomhall-Dillard, R. N. R., Gordon, R. G., & Wagner, V. A., MRS Proceedings, 1999, 606 | |||||||||||||
| Lithium tert-amyl(i-butyldimethylsilyl)amide | 145/0.1 | Broomhall-Dillard, R. N. R., Gordon, R. G., & Wagner, V. A., MRS Proceedings, 1999, 606 | |||||||||||||
| Lithium tert-amyl(n-propyldimethylsilyl)amide | 171/0.3 | Broomhall-Dillard, R. N. R., Gordon, R. G., & Wagner, V. A., MRS Proceedings, 1999, 606 | |||||||||||||
| Lithium bis(n-propyldimethylsilyl)amide | 130/0.15 | Broomhall-Dillard, R. N. R., Gordon, R. G., & Wagner, V. A., MRS Proceedings, 1999, 606 | |||||||||||||
| Lithium bis(i-butyldimethylsilyl)amide | 145/0.05 | Broomhall-Dillard, R. N. R., Gordon, R. G., & Wagner, V. A., MRS Proceedings, 1999, 606 | |||||||||||||
| Lithium tert-amyl(triethylsilyl)amide | 157/0.095 | Broomhall-Dillard, R. N. R., Gordon, R. G., & Wagner, V. A., MRS Proceedings, 1999, 606 | |||||||||||||
| Lithium bis(n-butyldimethylsilyl)amide | 145/0.085 | Broomhall-Dillard, R. N. R., Gordon, R. G., & Wagner, V. A., MRS Proceedings, 1999, 606 | |||||||||||||
| Lithium dimethylamide, (CH3)2NLi | 3585-33-9 | Catches fire spontaneously if exposed to air and in contact with water releases flammable gas. | https://pubchem.ncbi.nlm.nih.gov/compound/Lithium-dimethylamide | ||||||||||||
| Dicyclohexylamidolithium, C12H24Li2N | 4111-55-1 | High sublimation temperature of 250 °C at which it is also partly thermally decomposing. | 250 °C | Putkonen, M., Aaltonen, T., Alnes, M., Sajavaara, T., Nilsen, O., & Fjellvåg, H., Journal of Materials Chemistry, 2009, 19(46), 8767 | |||||||||||
| Li(acac), Lithium acetylacetonate, C5H7LiO2 | 18115-70-3 | Hygroscopic | Aerosol [1] | 250 °C | Methanol | [1] V. Bornand, Ph. Papet, E. Philippot, Thin Solid Films 1997, 304, 239. | |||||||||
| Lithium ethoxide, LiC2H5O | 2388-07-0 | Self heating and reacts violently with water. | Decomposes at 325 °C. LiOEt is insoluble in hydrocarbons, soluble in EtOH (125g/L), α = 6, 4 (MS), ΔHform = -108.6 | Powder subliming at 100 °C/vacuo, 150 °C /10-2 torr. | https://www.sigmaaldrich.com/catalog/product/aldrich/400203?lang=en | ||||||||||
| Lithium isopropoxide C3H7LiO | 2388-10-5 | "Sensitive to moisture and reacts with water. Material decomposes slowly in contact with moist air and rapidly in contact with water, possibly igniting. Avoid contact with moist air, water, acids, alcohols, ketones, esters, carbon dioxide, halogens." | Highly flammable, stable under nitrogen or argon in sealed containers |  | https://pubchem.ncbi.nlm.nih.gov/compound/Lithium-isopropoxide#section=Chemical-and-Physical-Properties | ||||||||||
| [Li(OtBu)]6, Lithium tert-butoxide, C4H9LiO | 1907-33-1 | Stable to light, heat, air, carbon dioxide and strong acids. Moisture sentitive, vigorous reaction to water. | 108-115 °C [1,2] | 283 °C | Soluble in toluene, hexane, tetrahydrofuran and methyl tert-butyl ether. | " [1] A. Dabirian, Y. Kuzminykh, S. C. Sandu, S. Harada, E. Wagner, P. Brodard, G. Benvenuti, S.Rushworth, P. Muralt, P. Hoffmann, Cryst. Growth Des. 2011, 1, 203. [2] A. Tanaka, K. Miyashita, T. Tashiro, M. Kimura, T. Sukegawa, J. Cryst. Growth 1995, 148, 324. [3] J. Hamalainen, J. Holopainen, F. Munnik, T. Hatanpaa, M. Heikkila, M. Ritala, and M. Leskela, J Electrochem Soc, 159, A259 (2012). [4] Sigma-Aldtritch" | |||||||||
| LiTa(OEt)6 | 127503-04-2 | The double alkoxides have sufficient stability using parent alcohol as solvent. Decomposes in contact with water. | The thermal stability and volatility vary with respect to the reaction in solid or liquid state. | 230/0.2 | https://www.chemicalbook.com/ChemicalProductProperty_EN_CB2739827.htm | ||||||||||
| lithium hexa-iso-propoxytantalate LiTa(i-OPr)6 | 160-180/0.1 | https://www.tms.org/pubs/journals/JOM/9710/Xu/Xu-9710.html | |||||||||||||
| LiTa(t-OBut)6 | 110-120/0.1 | https://www.tms.org/pubs/journals/JOM/9710/Xu/Xu-9710.html | |||||||||||||
| Lithium niobium ethoxide, LiNb(OC2H5)6 | Moisture Sensitive | Suyama, Y., Yamada, T., Hirano, Y., Takamura, K., & Takahashi, K. (2010). New Synthesis Process of Li, Na and K Niobates from Metal Alkoxides. Advances in Science and Technology, 63, 7–13. doi : 10.4028/www.scientific.net/ast.63.7 | |||||||||||||
| LiNb(i-OPr)6 | <140/0.2 | https://www.tms.org/pubs/journals/JOM/9710/Xu/Xu-9710.html | |||||||||||||
| LiNb(t-OBut)6 | 110-120/0.1 | https://www.tms.org/pubs/journals/JOM/9710/Xu/Xu-9710.html | |||||||||||||
| Sodium niobium ethoxide, NaNb(OC2H5)6 | Moisture Sensitive | Suyama, Y., Yamada, T., Hirano, Y., Takamura, K., & Takahashi, K. (2010). New Synthesis Process of Li, Na and K Niobates from Metal Alkoxides. Advances in Science and Technology, 63, 7–13. doi : 10.4028/www.scientific.net/ast.63.7 | |||||||||||||
| Sodium cyclopentadienide, C5H5Na | 4984-82-1 | In contact with water releases flammable gases which may ignite spontaneously. | Soluble in THF, benzene or liq. NH3 | "1. (a) Fischer, E. O.; Jira, R.; Hafner, K. Z. Naturforsch. 1953, 8b,(b) Fischer, E. O.; Hafner, W.; Stahl, H. O. Z. Anorg. Allg. Chem.1955, 282, 47. 2. Fehlhammer, W. P.; Herrmann, W. A.; O¨ fele, K. In Synthetic Methods of Organometallic and Inorganic Chemistry; Herrmann, W.A., Brauer, G., Eds.; Thieme: Stuttgart, 1997; Vol. 3, p 50. 3.https://spectrabase.com/spectrum/IMGzWBmNgJE. 4.https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-cyclopentadienide#section=GHS-Classification" | |||||||||||
| Sodium hexafluoroacetylacetonate, NaC5HF6O2 | 22466-49-5 | 25/10.3 |  | 230 °C | Soluble in water and warm methoxypropanol. | 1. Zh. Neorg. Khim. 41, 411, (1996). 2. Rec. Trav. Chim. 114, 242, (1995) | |||||||||
| Sodium 2,2,6,6-tetramethylheptane-3,5-dionate, Na(thd) | 22466-43-9 | Sublimes between 170 and 255 °C |  | M. Tiitta, M. Leskäla, E. Nykänen, P. Soinen, L. Niinstö, Thermochim. acta, 1995, 256 (1), 47-53 | |||||||||||
| Sodium 2,2,6,6-tetramethylheptane-3,5-dionate phenantroline, Na(thd)(phen) | Sublimes around 210 °C | D. Tsymbarenko, I. Korsakov, A. Mankevich, G. Girichev, E. Pelevina, A. Kaul, ECS Trans., 2009, vol.25, Iss.8, 633-638 | |||||||||||||
| Sodium 2,2,6,6-tetramethylheptane-3,5-dionate 2,2'-bipiridyne, Na(thd)(bipy) | It decomposes at 2 stages namely around 90 °C and 140 °C | D. Tsymbarenko, I. Korsakov, A. Mankevich, G. Girichev, E. Pelevina, A. Kaul, ECS Trans., 2009, vol.25, Iss.8, 633-638 | |||||||||||||
| Sodium-niobium hexakis(isopropoxide), NaNb(OiPr)6 | 110-120/0.1 | ||||||||||||||
| Sodium bis(n-propyldimethylsilyl)amide | 213/0.3 | Broomhall-Dillard, R. N. R., Gordon, R. G., & Wagner, V. A., MRS Proceedings, 1999, 606 | |||||||||||||
| Sodium bis(i-butyldimethylsilyl)amide | 189/0.08 | Broomhall-Dillard, R. N. R., Gordon, R. G., & Wagner, V. A., MRS Proceedings, 1999, 606 | |||||||||||||
| Sodium bis(n-butyldimethylsilyl)amide | 231/0.5 | Broomhall-Dillard, R. N. R., Gordon, R. G., & Wagner, V. A., MRS Proceedings, 1999, 606 | |||||||||||||
| Sodium bis(n-hexyldimethylsill)amide | 265/0.3 | Broomhall-Dillard, R. N. R., Gordon, R. G., & Wagner, V. A., MRS Proceedings, 1999, 606 | |||||||||||||
| Sodium Tert Butoxide, NaOC(CH3)3 | 865-48-5 | Stable at room temperature. Decomposes at 300 °C; stable under N2 or Ar in sealed container and decomposes slowly in contact with moist air and violently in contact with water. [1] | At 300 °C [1] | sublimation: 254 °C [2] (atmospheric pressure) | Information not available | Information not available | 263 °C [3] | "• 30 g/L at 20 °C Medium: tert-butyl alcohol • 70 g/L at 20 °C Medium: Toluene • 130 g/L at 20 °C Medium: Hexane • 380 g/L at 20 °C Medium: Tetrahydrofuran • 50 g/L at 20 °C Medium: xylene • 110 g/L at 20 °C Medium: octane • 220 g/L at 20 °C Medium: Diethyl ether • 450 g/L at 20 °C Medium: Dimethylformamide | ": [1] https://www.nwmissouri.edu/naturalsciences/sds/s/Sodium%20tert-butoxide.pdf: [2] https://www.albemarle.com/storage/components/T401225.PDF: [3] Simone Manzini, Núria Huguet, Oliver Trapp, Rocco A. Paciello, Thomas Schaub; "Synthesis of acrylates from olefins and CO2 using sodium alkoxides as bases" Catalysis Today, Volume 281, Part 2, 2017, Pages 379–386, ISSN 0920-5861 | ||||||
| Potassium-niobium hexakis(ethoxide), KNb(OEt)6 | 200/0.8 | Suyama, Y., Yamada, T., Hirano, Y., Takamura, K., & Takahashi, K. (2010). New Synthesis Process of Li, Na and K Niobates from Metal Alkoxides. Advances in Science and Technology, 63, 7–13. doi : 10.4028/www.scientific.net/ast.63.7 | |||||||||||||
| Potassium tert-butoxide (KOtBu) C4H9KO | 865-47-4 | Sublimes at temperature of 220 °C at pressure of 1 Torr [1] | NA | 220/1 | 256 °C-258 °C [2] | Soluble in hexane, toluene, diethyl ether and tetrahydrofuran. | [1] Feuer et al.Journal of the American Chemical Society1956vol. 78p. 4364,4367 [2] https://www.sigmaaldrich.com/catalog/product/aldrich/156671?lang=de®ion=DE [3] Labbow, R., Michalik, D., Reiß, F., Schulz, A. and Villinger, A., 2016. Isolation of Labile Pseudohalogen NSO Species. Angewandte Chemie International Edition, 55(27), pp. 7680–7684. | ||||||||
| Potassium 2,2,6,6-tetramethylheptane-3,5-dionate, K(thd), K(tmhd), K(dpm), C11H19KO2 | 22441-14-1 | Hygroscopic |  | 195 °C | 1. Onoe, A., Tasaki, Y., & Chikuma, K. (2005). Anomalous evaporation characteristics of vitrificated K(DPM) and stable gas supply using disk-shaped K(DPM) precursors for metalorganic chemical vapor deposition. Journal of Crystal Growth, 277(1-4), 546–554. doi : 10.1016/j.jcrysgro.2005.01.077 2. www.molbase.com | ||||||||||
| Potassium 2,2,6,6-tetramethylheptane-3,5-dionate phenantroline, K(thd)(phen) | 320-330 °C | Oligomerizes with n up to 7 | D. Tsymbarenko, I. Korsakov, A. Mankevich, G. Girichev, E. Pelevina, A. Kaul, ECS Trans., 2009, vol.25, Iss.8, 633-638 | ||||||||||||
| Bi(phenyl)3,Triphenylbismuth(III), (C6H5)3Bi | 603-33-8 | No specific storage condition | 76-80 °C | [1] Sigma | |||||||||||
| Fe(tmhd)3,Tris(2,2,6,6-tetramethyl-3,5-heptanedionato)iron(III), Fe(C11H19O2)3 | 14876-47-2 |  | 164 °C (Atm) (STREM); 179-185 °C (lit.) (Sigma) | [1] Sigma [2] Strem | |||||||||||
| Ni(hfa)2tmeda | Evaporation occurs in the 120–200 _C temperature range, with about 2%residue at 350 _C (Atm under N2)" | 120–200 °C (Atm pressure under N2) | 106,7°C | [3] Sergio Battiato, Maria M. Giangregorio, Maria R. Catalano, Raffaella Lo Nigro, Maria Losurdo and Graziella Malandrino; RSC Adv., 2016, 6, 30813–30823 | |||||||||||
| Ni(tta)2tmeda | evaporated quantitatively in the 200–330 _C range, with less than 2% residue le at 350_°C. (Atm under N2) | 2774(2) A˚ 3, Z = 4, Dc = 1.478 g cm−3 | 147–149°C | to request | to request | [3] Sergio Battiato, Maria M. Giangregorio, Maria R. Catalano, Raffaella Lo Nigro, Maria Losurdo and Graziella Malandrino; RSC Adv., 2016, 6, 30813–30823 | |||||||||
| Ni(tmhd)2,Nickel(II) bis(2,2,6,6-tetramethyl-3,5-heptanedionate), Ni(OCC(CH3)3CHCOC(CH3)3)2 | 14481-08-4 | 219-223°C (Atm) | Maria Losurdo and Graziella Malandrino; RSC Adv., 2016, 6, 30813–30823 [4] Malandrino, Graziella & M S Perdicaro, Laura & Condorelli, Giuseppe & Fragalà, Ignazio & Rossi, Patrizia & Dapporto, Paolo. (2006). Dalton transactions (Cambridge, England : 2003). 8. 1101-6. 10.1039/b511317b. | ||||||||||||
| Ni(acac)2, Nickel(II) acetylacetonate, Ni(C5H7O2)2 | 3264-82-2 | 230 - 240°C | ethers and aromatic and halogenated hydrocarbons | [1] SIGMA [4] Malandrino, Graziella & M S Perdicaro, Laura & Condorelli, Giuseppe & Fragalà, Ignazio & Rossi, Patrizia & Dapporto, Paolo. (2006). Dalton transactions (Cambridge, England : 2003). 8. 1101-6. 10.1039/b511317b. [6] A. Pande, Synlett, 2005, 6, 1042–1043 | |||||||||||
| La(hfa)3diglyme | nonhygroscopic, can be handled in air | "TGA, 10 ""Clmin under N2) reveal that the sublimation processes takes place in the 115-295°C (residue = 2% to 300°C)" | 74-76 °C | Ethanol, chloroform, acetone, pentane, toluene and slightly soluble in cyclohexane | [7] Graziella Malandrino, Rosalia Licata, Francesco Castelli, Ignazio L. Fragala, and Cristiano Benelli Inorganic Chemistry 1995 34 (25), 6233-6234" | ||||||||||
| Nb(THD)4, Niobium tetrakis(2,2,6,6-tetramethylheptane-3,5-dionate), C44H76NbO8 | 41706-15-4 | Air and moisture stable, insoluble in water. | Under atmospheric pressure and inert atmosphere Li(thd) evaporates completely before ≈270 °C without decomposition. Heating of Nb(thd)4 under similar conditions results in a solid residue of ≈7% what shows that evaporation and decomposition of this compound goes simultaneously (full decomposition of Nb(thd)4 to Nb2O5 should leave 16.1% residue). [1] | 219-220 °C | 1,2-dimethoxyethane | [1] S. Margueron, A. Bartasyte, V. Plausinaitiene, A. Abrutis, P. Boulet, V. Kubilius, Z. Saltyte, Proc. SPIE 2013, 8626, 862612. | |||||||||
| Nb(thd)2Cl3, Bis-dipivaloylmethanate niobium N-chloride, C4H10Cl3NbO2 | 110615-13-9 | Air sensitive. Hydrolyses readily. | 170 °C [1] | 230 °C | [1] S. Jung, N. Imaish, Korean, J. Chem. Eng. 1999, 16, 229. [2] Sigma-Aldritch | ||||||||||
| Niobium pentakis(methoxide), Nb(OMe)5 | Low volatility | 200 °C [1] | [1] B. J. Curtis, H. R. Brunner, Mater. Res. Bull. 1975, 10, 515. | ||||||||||||
| Nb(OEt)5 , Niobium pentaethoxide, C10H25NbO5 | 3236-82-6 | Air and moisture sensitive. Incompatible with strong acids and strong oxidizing agents. | 135-145 °C [1] 100-120 °C [2] | 5-6 °C | Dry touluene, ethanol. | [1] Y. Sakashita, H. Segawa, J. Appl. Phys. 1995, 77, 5995 [2] Y. Akiyama, K. Shitanaka, H. Murakami, Y. S. Shin, M. Yoshida, N. Imaishi, Thin Solid Films 2007, 515, 4975. [3] Sigma-Aldritch | |||||||||
| Niobium ethoxide, Nb(OCH2CH3)5 | 3236-82-6 | Stable at room temperature. Stable under N2 or Ar in sealed container and decomposes quickly in contact with moist air. Reacts with water. [1] | At 325-350 °C [2] | Information not available | 21.5 kPa at 500 K [3] | At 325-350 °C [2] | Dimer | At 5 °C [4] | Soluble in organic solvents. Decomposes in water.Miscible with organic solvents [4] | : [1] https://www.gelest.com/wp-content/uploads/product_msds/AKN590-msds.pdf: [2] Rahtu, Antti (2002). Atomic Layer Deposition of High Permittivity Oxides: Film Growth and In Situ Studies (Thesis). University of Helsinki. ISBN 952-10-0646-3: [3] Niobium(V) ethoxide: [4] Cai Ya-nan, Yang Sheng-hai, Jin Sheng-ming, Yang Hai-ping, Hou Guo-feng, Xia Jiao-yun,"Electrochemical synthesis, characterization and thermal properties of niobium ethoxide"; J. Cent. South Univ. Technol. (2011) 18: 73−77: [5] https://www.chemicalbook.com/ChemicalProductProperty_EN_CB3759592.htm | |||||
| Pentakis(dimethylamino)tantalum(V), Ta(N(CH3)2)5 | 19824-59-0 | Reacts violently with water |  | 100oC | https://www.sigmaaldrich.com/catalog/product/aldrich/496863?lang=en | ||||||||||
| Tantalum(V) ethoxide, Ta(OC2H5)5 | 6074-84-6 | 21oC | https://www.sigmaaldrich.com/catalog/product/aldrich/760404?lang=en | ||||||||||||
| Tris(diethylamido)(tert-butylimido)tantalum(V), (CH3)3CNTa(N(C2H5)2)3 | 169896-41-7 | Reacts violently with water |  | https://www.sigmaaldrich.com/catalog/product/aldrich/521280?lang=en | |||||||||||
| Tris(ethylmethylamido)(tert-butylimido)tantalum(V), C13H33N4Ta | 511292-99-2 | Reacts violently with water |  | https://www.sigmaaldrich.com/catalog/product/aldrich/j100043?lang=en | |||||||||||
| Cesium-yttrium tetrakis (1,1,1-trifluoro -5,5-dimethylhexane-2,4-dionate) C32H40O8F12CsY | Vikulova, E. S., Zherikova, K. V., Zelenina, L. N., Trubin, S. V., Sysoev, S. V., Semyannikov, Asanov I. V., Morozova N. B., Igumenov, I. K., J. Chem. Thermodynamics 69 (2014) 137–144 | ||||||||||||||
| Cesium-yttrium tetrakis (2,2,6,6-tetramethyl-3,5-heptanedionate) | sublimes at 230 °C | A.A. Vorobjev, Course Thesis, http://www.bibliofond.ru/view.aspx?id=555884 | |||||||||||||
| Cesium-yttrium tetrakis (hexafluoracetylacetonate) CS[Y(CF3COCHCOCF3)4] | M. J. Bennett, F. A. Cotton, P. Legzdins, S. J. Lippard, Inorg. Chem., 1968, 7 (9), pp 1770–1776, | ||||||||||||||
| Cesium-lantanum tetrakis (hexafluoracetylacetonate) | C, E. Higgins, J. Inorg. Nucl. Chem., 1973, Vol 35, Iss. 6p. 1941–1944 | ||||||||||||||
| Cesium-europium tetrakis (hexafluoracetylacetonate) | [i] C, E. Higgins, J. Inorg. Nucl. Chem., 1973, Vol 35, Iss. 6p. 1941–1944 [ii] J. H. Burns, M. D. Danford, Inorg. Chem., 1969, 8 (8), pp 1780–1784, doi : 10.1021/ic50078a048, | ||||||||||||||
| Rubidium acetylacetonate RbC5H7O2 | 66169-93-5 | melting point: 200 °C | C.R. Bhattacharjee, M. Bhattacharjee; M.K. Chaudhuri, H. Sangchungnunga, J. Chem. Res. Synopses, 1991, no9, pp. 250–251 | ||||||||||||
| Rubidium 2,2,6,6-tetramethylheptane-3,5-dionate C11H19O2Rb | 166439-15-2 | Rb(thd) was found to be completely insoluble in supercritical CO2 (0 mol/L) under these conditions: 100-200bar/ 60 °C | O. Aschenbrenner, S. Kemper, N. Dahmen, K. Schaber, E. Dinjus, J. Supercritical Fluids, 2007, Vol.41, Iss.2, p. 179–186 | ||||||||||||
| Rubidium trimethysilyloxide | sublimes at 80 °C/ 10-6 Torr and decomposes at 140 °C | ||||||||||||||
| Rubidium isopropoxide Rb(OiPr) | sublimes under deep vacuum (10-6 Torr) despite of its polymeric nature, surprisingly it sublimes at higher temperature (200 °C) | ||||||||||||||
| Rubidium tert-butoxide Rb(OtBu) | sublimable at 185-200 °C/ 10-2 Torr. | M.H. Chisholm, S.R. Drake, A.A. Naiini, W.E. Streib, Polyhedron, 1991, Vol. 10, Iss.3, p. 337–345 | |||||||||||||
| Dimethyl aluminum acetylacetonate (CH3)2Al(C5H7O2) | G. A. Battiston, G. Carta, G. Cavinato, R. Gerbasi, M. Porchia G. Rossetto, Chem.Vapor.Dep., 2001, Vol.7, Issue2, Pages 69–74 | ||||||||||||||
| Diethyl aluminum acetylacetonate | G. A. Battiston, G. Carta, G. Cavinato, R. Gerbasi, M. Porchia G. Rossetto, Chem.Vapor.Dep., 2001, Vol.7, Issue2, Pages 69–74 | ||||||||||||||
| Diisobutyl aluminum acetylacetonate | G. A. Battiston, G. Carta, G. Cavinato, R. Gerbasi, M. Porchia G. Rossetto, Chem.Vapor.Dep., 2001, Vol.7, Issue2, Pages 69–74 | ||||||||||||||
| Dimethylamine alane NH(CH3)2 · AlH3 | |||||||||||||||
| Trimethylamine alane AlH3 · N(CH3)3 | 16842-00-5 | /www.sigmaaldrich.com/catalog/product/aldrich/455792 | |||||||||||||
| Triethylamine alane | Triethylamine alane (TEAA) decomposes on an Al(111) single crystal surface at temperatures above - 310 K | Dubois, L. H., Zegarski, B. R., Gross, M. E., & Nuzzo, R. G. 1991, Surface Science, 244(1-2), 89–95. | |||||||||||||
| Dimethylethylamine alane C2H5N(CH3)2 · AlH3 | 124330-23-0 | www.sigmaaldrich.com/catalog/product/aldrich/400386?lang=it®ion=IT | |||||||||||||
| Dimethylaluminum hydride (CH3)2AlH | 865-37-2 | www.americanelements.com/dimethylaluminum-hydride-865-37-2#:~:text=Dimethylaluminum%20Hydride%20is%20one%20of,portable%20sources%20of%20hydrogen%20gas. | |||||||||||||
| Di-iso-butylaluminum hydride [(CH3)2CHCH2]2AlH | 1191-15-7 | /www.sigmaaldrich.com/catalog/product/aldrich/190306 | |||||||||||||
| Calcium bis(cyclopentadienyl) (calcocene) C10H10Ca | PubChem CID: 100977887 | pubchem.ncbi.nlm.nih.gov/compound/Bis_2_4-cyclopentadienyl_-calcium | |||||||||||||
| Calcium bis(isopropylcyclopentadienyl) [(C3H7)3C5H2]2Ca · (CH3OCH2)2 | ereztech.com/product/bistri-isopropylcyclopentadienylcalcium-12-dimethoxyethane-adduct-n-a/ | ||||||||||||||
| calcium bis[bis(trimethylsilyl)amide C12H36CaN2Si4 | ChemSpider ID: 9243563 | /www.chemspider.com/Chemical-Structure.9243563.html | |||||||||||||
| calcium bis[bis(trimethylsilyl)amide dimethoxyethane | Matthias. Westerhausen, Inorganic Chemistry 1991 30 (1), 96-101 | ||||||||||||||
| calcium bis[bis(trimethylsilyl)amide tetrahydrofuran | Matthias. Westerhausen, Inorganic Chemistry 1991 30 (1), 96-101 | ||||||||||||||
| Calcium bis(acetylacetonate) Ca(CH3COCHCOCH3)2 | 19372-44-2 | Melting point >280 °C | www.americanelements.com/calcium-acetylacetonate-19372-44-2 | ||||||||||||
| Calcium bis(hexafluoracetylacetonate) tetraglyme | [i] Malandrino, G., Castelli, F., & Fragalà, I. L., Inorganica Chimica Acta, 1994, 224(1-2), 203–207. [ii] D.M. Tsymbarenko et al. / Polyhedron 134 (2017) 246–256 | ||||||||||||||
| Calcium bis(2,2,6,6-tetramethyl-3,5-heptanedonate) Ca(OCC(CH3)3CHCOC(CH3)3)2 | 118448-18-3 | 221-224 °C | www.sigmaaldrich.com/catalog/product/aldrich/362956?lang=it®ion=IT | ||||||||||||
| Calcium 1,1,1,2,2,3,3,7,7,8,8,9,9,9-tetradecafluorononane-4,6-dionate monohydrate | Simon C. Thompson, David J. Cole-hamilton, Douglas D. Gilliland, Michael L. Hitchman, John C. Barnes, Advanced Materials for Optics and Electronics, Volume 1, Issue 2, pages 81–97, April 1992 | ||||||||||||||
| Calcium bis(tert-butyl)dimethylketiminate | El-Kaderi, H. M., Heeg, M. J., & Winter, C. H., Organometallics, 23(21), 2004, 4995–5002. | ||||||||||||||
| Calcium bis(isopropyl)dimethylketiminate | El-Kaderi, H. M., Heeg, M. J., & Winter, C. H., Organometallics, 23(21), 2004, 4995–5002. | ||||||||||||||
| Chromium (III) 2-ethylhexanoate C24H45CrO6 | 3444-17-5 | www.chemicalbook.com/ChemicalProductProperty_EN_CB5738861.htm | |||||||||||||
| Chromium (III) diethyldithiocarbamate | Sedlacek, J., Martins, L. M. D. R. S., Danek, P., Pombeiro, A. J. L., & Cvek, B., Journal of Applied Biomedicine, 2014, 12(4), | ||||||||||||||
| Chromium tris(acetylacetonate) Cr(C5H7O2)3 | 21679-31-2 | www.sigmaaldrich.com/catalog/product/aldrich/574082?lang=it®ion=IT | |||||||||||||
| Chromium tris(trifluoroacetylacetonate) Cr(C5H4F3O2)3 | 14592-89-3 | /www.sigmaaldrich.com/catalog/product/aldrich/495697?lang=it®ion=IT | |||||||||||||
| Chromium tris(hexafluoroacetylacetonate) Cr(CF3COCHCOCF3)3 | 14592-80-4 | www.americanelements.com/chromium-iii-hexafluoroacetylacetonate-14592-80-4 | |||||||||||||
| hromium tris(2,2,6,6-tetramethyl-3,5-heptanedionate) Cr(OCC(CH3)3CHCOC(CH3)3)3 | 14434-47-0 | www.sigmaaldrich.com/catalog/product/aldrich/468223?lang=it®ion=IT | |||||||||||||
| Dysprosium tris(acetylacetonate) Dy(C5H7O2)3• xH2O | 18716-76-2 | www.americanelements.com/dysprosium-acetylacetonate-18716-76-2#:~:text=Dysprosium%20Acetylacetonate%20is%20one%20of,energy%20and%20water%20treatment%20applications. | |||||||||||||
| Dysprosium tris(2,2,6,6-tetramethyl-3,5-heptanedionate) Dy(C11H19O2)3 | 15522-69-7 | www.americanelements.com/tris-2-2-6-6-tetramethyl-3-5-heptanedionato-dysprosium-iii-15522-69-7 | |||||||||||||
| Dysprosium tris(6-ethyl-2,2-dimethyl-3,5-decanedionate) Dy(OCC(CH3)3CHCOCF2CF2CF3)3 | 18323-98-3 | www.sigmaaldrich.com/catalog/product/aldrich/237280?lang=it®ion=IT | |||||||||||||
| Dysprosium tris(isopropoxide) Dy(OC3H7)3 | 6742-68-3 | www.americanelements.com/dysprosium-iii-isopropoxide-6742-68-3 | |||||||||||||
| Dysprosium tris(1-methoxy-2-methyl-2-propanolate) | Van Elshocht, S., Lehnen, P., Seitzinger, B., Abrutis, A., Adelmann, C., Brijs, B., ... Heyns, M., Journal of The Electrochemical Society, 153(9), 2006 |