
Gene therapy is a medical technology which aims to produce a therapeutic effect through the manipulation of gene expression or through altering the biological properties of living cells.

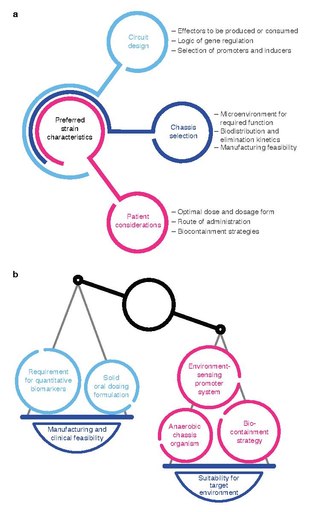
Synthetic biology (SynBio) is a multidisciplinary field of science that focuses on living systems and organisms, and it applies engineering principles to develop new biological parts, devices, and systems or to redesign existing systems found in nature.
Cancer research is research into cancer to identify causes and develop strategies for prevention, diagnosis, treatment, and cure.

Cancer immunotherapy is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.
Alkylphosphocholines are phospholipid-like molecules that have been synthesised, which have remarkable biological and therapeutic activities. They are phosphocholine esters of aliphatic long chain alcohols differing in chain length, unsaturation and position of the cis-double bond.
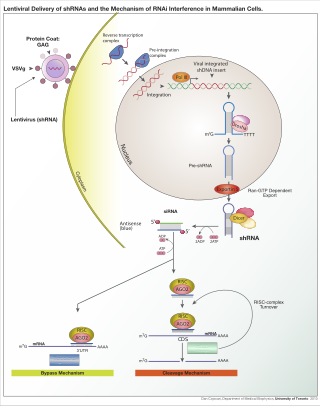
A short hairpin RNA or small hairpin RNA is an artificial RNA molecule with a tight hairpin turn that can be used to silence target gene expression via RNA interference (RNAi). Expression of shRNA in cells is typically accomplished by delivery of plasmids or through viral or bacterial vectors. shRNA is an advantageous mediator of RNAi in that it has a relatively low rate of degradation and turnover. However, it requires use of an expression vector, which has the potential to cause side effects in medicinal applications.

Bexarotene, sold under the brand Targretin, is an antineoplastic (anti-cancer) agent used for the treatment of cutaneous T cell lymphoma (CTCL). It is a third-generation retinoid.
Microbubbles (MBs) are bubbles smaller than one hundredth of a millimetre in diameter, but larger than one micrometre. They have widespread application in industry, medicine, life science, and food technology. The composition of the bubble shell and filling material determine important design features such as buoyancy, crush strength, thermal conductivity, and acoustic properties.
Wafik El-Deiry is an American physician and cancer researcher who is the Associate Dean for Oncologic Sciences at the Warren Alpert Medical School, Brown University, Director of the Cancer Center at Brown University, and the Director of the Joint Program in Cancer Biology at Brown University and its affiliated hospitals. He was previously deputy director of Translational Research at Fox Chase Cancer Center, where he was also co-Leader of the Molecular Therapeutics Program.
Alternating electric field therapy, sometimes called tumor treating fields (TTFields), is a type of electromagnetic field therapy using low-intensity, intermediate frequency electrical fields to treat cancer. A TTField-generating device manufactured by the Israeli company Novocure is approved in the United States and Europe for the treatment of newly diagnosed and recurrent glioblastoma multiforme (GBM), and is undergoing clinical trials for several other tumor types. Despite earning regulatory approval, the efficacy of this technology remains controversial among medical experts.

Sonodynamic therapy (SDT) is a noninvasive treatment, often used for tumor irradiation, that utilizes a sonosensitizer and the deep penetration of ultrasound to treat lesions of varying depths by reducing target cell number and preventing future tumor growth. Many existing cancer treatment strategies cause systemic toxicity or cannot penetrate tissue deep enough to reach the entire tumor; however, emerging ultrasound stimulated therapies could offer an alternative to these treatments with their increased efficiency, greater penetration depth, and reduced side effects. Sonodynamic therapy could be used to treat cancers and other diseases, such as atherosclerosis, and diminish the risk associated with other treatment strategies since it induces cytotoxic effects only when externally stimulated by ultrasound and only at the cancerous region, as opposed to the systemic administration of chemotherapy drugs.

Sanjiv Sam Gambhir was an American physician–scientist. He was the Virginia and D.K. Ludwig Professor in Cancer Research, Chairman of the Department of Radiology at Stanford University School of Medicine, and a professor by courtesy in the departments of Bioengineering and Materials Science and Engineering at Stanford University. Additionally, he served as the Director of the Molecular Imaging Program at Stanford (MIPS), Canary Center at Stanford for Cancer Early Detection and the Precision Health and Integrated Diagnostics Center (PHIND). He authored 680 publications and had over 40 patents pending or granted. His work was featured on the cover of over 25 journals including the Nature Series, Science, and Science Translational Medicine. He was on the editorial board of several journals including Nano Letters, Nature Clinical Practice Oncology, and Science Translational Medicine. He was founder/co-founder of several biotechnology companies and also served on the scientific advisory board of multiple companies. He mentored over 150 post-doctoral fellows and graduate students from over a dozen disciplines. He was known for his work in molecular imaging of living subjects and early cancer detection.
Tal Danino is a synthetic biologist and Associate Professor of Biomedical Engineering at Columbia University.
David A. Scheinberg is an American physician, scientist, drug developer, and entrepreneur, who is currently Vincent Astor Chair, and Chairman of the Molecular Pharmacology Program at Memorial Sloan Kettering Cancer Center (MSK). He is a pioneer and inventor of targeted alpha particle therapies and alpha particle generators for use in patients with cancer.
Luis Alberto Diaz, Jr. is the Head of the Division of Solid Tumor Oncology in Memorial Sloan Kettering’s Department of Medicine.
Evolutionary therapy is a subfield of evolutionary medicine that utilizes concepts from evolutionary biology in management of diseases caused by evolving entities such as cancer and microbial infections. These evolving disease agents adapt to selective pressure introduced by treatment, allowing them to develop resistance to therapy, making it ineffective.
Bacterial therapy is the therapeutic use of bacteria to treat diseases. Bacterial therapeutics are living medicines, and may be wild type bacteria or bacteria that have been genetically engineered to possess therapeutic properties that is injected into a patient. Other examples of living medicines include cellular therapeutics, activators of anti-tumor immunity, or synergizing with existing tools and approaches. and phage therapeutics, or as delivery vehicles for treatment, diagnosis, or imaging, complementing or synergizing with existing tools and approaches.
Tumor homing bacteria is a group of facultative or obligate anaerobic bacteria that are able to target cancerous cells in the body, suppress tumor growth and survive in the body for a long time even after the infection. When this type of bacteria is administered into the body it migrates to the cancerous tissues and starts to grow, then deploys distinct mechanisms to destroy solid tumors. Each bacteria species uses a different process to eliminate the tumor. Some common tumor homing bacteria include Salmonella, Clostridium, Bifidobacterium, Listeria, and Streptococcus. The earliest research of this type of bacteria was highlighted in 1813 when scientists began observing that patients that had gas gangrene, an infection caused by the bacteria Clostridium, were able to have tumor regressions.

Michel Sadelain is an immunologist and genetic engineer at Memorial Sloan Kettering Cancer Center, New York, New York, where he holds the Steve and Barbara Friedman Chair. He is the founding director of the Center for Cell Engineering and the head of the Gene Transfer and Gene Expression Laboratory. He is a member of the department of medicine at Memorial Hospital and of the immunology program at the Sloan Kettering Institute. He is best known for his major contributions to T cell engineering and chimeric antigen receptor (CAR) therapy, an immunotherapy based on the genetic engineering of a patient's own T cells to treat cancer.
RNA therapeutics are a new class of medications based on ribonucleic acid (RNA). Research has been working on clinical use since the 1990s, with significant success in cancer therapy in the early 2010s. In 2020 and 2021, mRNA vaccines have been developed globally for use in combating the coronavirus disease. The Pfizer–BioNTech COVID-19 vaccine was the first mRNA vaccine approved by a medicines regulator, followed by the Moderna COVID-19 vaccine, and others.














