
An oxide is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– ion with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 that protects the foil from further oxidation.

Sulfur monoxide is an inorganic compound with formula SO. It is only found as a dilute gas phase. When concentrated or condensed, it converts to S2O2 (disulfur dioxide). It has been detected in space but is rarely encountered intact otherwise.
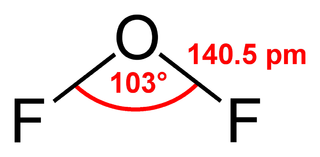
Oxygen difluoride is a chemical compound with the formula OF2. As predicted by VSEPR theory, the molecule adopts a bent molecular geometry. It is strong oxidizer and has attracted attention in rocketry for this reason. With a boiling point of −144.75 °C, OF2 is the most volatile (isolable) triatomic compound. The compound is one of many known oxygen fluorides.
Sulfur oxide refers to many types of sulfur and oxygen containing compounds such as SO, SO2, SO3, S7O2, S6O2, S2O2, etc.

Tetrasulfur tetranitride is an inorganic compound with the formula S4N4. This gold-poppy coloured solid is the most important binary sulfur nitride, which are compounds that contain only the elements sulfur and nitrogen. It is a precursor to many S-N compounds and has attracted wide interest for its unusual structure and bonding.
Sulfur compounds are chemical compounds formed the element sulfur (S). Common oxidation states of sulfur range from −2 to +6. Sulfur forms stable compounds with all elements except the noble gases.

Sulfur dichloride is the chemical compound with the formula SCl2. This cherry-red liquid is the simplest sulfur chloride and one of the most common, and it is used as a precursor to organosulfur compounds. It is a highly corrosive and toxic substance, and it reacts on contact with water to form chlorine-containing acids.
A polysulfane is a chemical compound of formula H2Sn, where n > 1. Compounds containing 2 – 8 sulfur atoms have been isolated, longer chain compounds have been detected, but only in solution. H2S2 is colourless, higher members are yellow with the colour increasing with the sulfur content.In the chemical literature the term polysulfanes is sometimes used for compounds containing −(S)n−, e.g. organic polysulfanes R1−(S)n−R2.

The element sulfur exists as many allotropes. In number of allotropes, sulfur is second only to carbon. In addition to the allotropes, each allotrope often exists in polymorphs delineated by Greek prefixes.

Disulfur is the diatomic molecule with the formula S2. It is analogous to the dioxygen molecule but rarely occurs at room temperature. This violet gas is the dominant species in hot sulfur vapors. S2 is one of the minor components of the atmosphere of Io, which is predominantly composed of SO2. The instability of S2 is usually described in the context of the double bond rule.
Sulfur mononitride is an inorganic compound with the molecular formula SN. It is the sulfur analogue of and isoelectronic to the radical nitric oxide, NO. It was initially detected in 1975, in outer space in giant molecular clouds and later the coma of comets. This spurred further laboratory studies of the compound. Synthetically, it is produced by electric discharge in mixtures of nitrogen and sulfur compounds, or combustion in the gas phase and by photolysis in solution.

Disulfur monoxide or sulfur suboxide is an inorganic compound with the formula S2O, one of the lower sulfur oxides. It is a colourless gas and condenses to give a roughly dark red coloured solid that is unstable at room temperature.
In organometallic chemistry, metal sulfur dioxide complexes are complexes that contain sulfur dioxide, SO2, bonded to a transition metal. Such compounds are common but are mainly of theoretical interest. Historically, the study of these compounds has provided insights into the mechanisms of migratory insertion reactions.

The S3 molecule, known as trisulfur, sulfur trimer, thiozone, or triatomic sulfur, is a cherry-red allotrope of sulfur. It comprises about 10% of vaporised sulfur at 713 K and 1,333 Pa. It has been observed at cryogenic temperatures as a solid. Under ordinary conditions it converts to cyclooctasulfur.

Disulfur dioxide, dimeric sulfur monoxide or SO dimer is an oxide of sulfur with the formula S2O2. The solid is unstable with a lifetime of a few seconds at room temperature.

Compounds of lead exist with lead in two main oxidation states: +2 and +4. The former is more common. Inorganic lead(IV) compounds are typically strong oxidants or exist only in highly acidic solutions.
The chalcogens react with each other to form interchalcogen compounds.

Thiosulfurous acid is a hypothetical chemical compound with the formula HS−S(=O)−OH or HO−S(=S)−OH. Attempted synthesis leads to polymers. It is a low oxidation state (+1) sulfur acid. It is the Arrhenius acid for disulfur monoxide. Salts derived from thiosulfurous acid, which are also unknown, are named "thiosulfites" or "sulfurothioites". The ion is S=SO2−
2.

Sulfoxylic acid (H2SO2) (also known as hyposulfurous acid or sulfur dihydroxide) is an unstable oxoacid of sulfur in an intermediate oxidation state between hydrogen sulfide and dithionous acid. It consists of two hydroxy groups attached to a sulfur atom. Sulfoxylic acid contains sulfur in an oxidation state of +2. Sulfur monoxide (SO) can be considered as a theoretical anhydride for sulfoxylic acid, but it is not actually known to react with water.
Dihydroxydisulfane or hypodithionous acid is a reduced sulfur oxyacid with sulfur in a formal oxidation state of +1, but the valence of sulfur is 2. The structural formula is HO−S−S−OH, with all atoms arranged in a chain. It is an isomer of thiosulfurous acid but is lower in energy. Other isomers include HOS(=O)SH, HOS(=S)OH, and HS(=O)2SH. Disulfur monoxide, S2O, can be considered as the anhydride. Unlike many of these other reduced sulfur acids, dihydroxydisulfane can be formed in a pure state by reacting hydrogen sulfide with sulfur dioxide at −70 °C in dichlorodifluoromethane.















