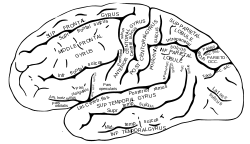
The visual cortex of the brain is the area of the cerebral cortex that processes visual information. It is located in the occipital lobe. Sensory input originating from the eyes travels through the lateral geniculate nucleus in the thalamus and then reaches the visual cortex. The area of the visual cortex that receives the sensory input from the lateral geniculate nucleus is the primary visual cortex, also known as visual area 1 (V1), Brodmann area 17, or the striate cortex. The extrastriate areas consist of visual areas 2, 3, 4, and 5.
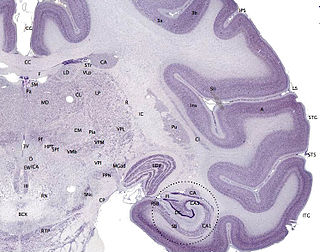
The cerebral cortex, also known as the cerebral mantle, is the outer layer of neural tissue of the cerebrum of the brain in humans and other mammals. The cerebral cortex mostly consists of the six-layered neocortex, with just 10% consisting of allocortex. It is separated into two cortices, by the longitudinal fissure that divides the cerebrum into the left and right cerebral hemispheres. The two hemispheres are joined beneath the cortex by the corpus callosum. The cerebral cortex is the largest site of neural integration in the central nervous system. It plays a key role in attention, perception, awareness, thought, memory, language, and consciousness.

The parietal lobe is one of the four major lobes of the cerebral cortex in the brain of mammals. The parietal lobe is positioned above the temporal lobe and behind the frontal lobe and central sulcus.

The precuneus is the portion of the superior parietal lobule on the medial surface of each brain hemisphere. It is located in front of the cuneus. The precuneus is bounded in front by the marginal branch of the cingulate sulcus, at the rear by the parietooccipital sulcus, and underneath by the subparietal sulcus. It is involved with episodic memory, visuospatial processing, reflections upon self, and aspects of consciousness.

The central sulcus is a sulcus, or groove, in the cerebral cortex in the brains of vertebrates. Also called the central fissure, or the fissure of Rolando or the Rolandic fissure, after Luigi Rolando. It is sometimes confused with the longitudinal fissure.

The occipital lobe is one of the four major lobes of the cerebral cortex in the brain of mammals. The name derives from its position at the back of the head, from the Latin ob, "behind," and caput, "the head."

Brodmann area 10 is the anterior-most portion of the prefrontal cortex in the human brain. BA10 was originally defined broadly in terms of its cytoarchitectonic traits as they were observed in the brains of cadavers, but because modern functional imaging cannot precisely identify these boundaries, the terms anterior prefrontal cortex, rostral prefrontal cortex and frontopolar prefrontal cortex are used to refer to the area in the most anterior part of the frontal cortex that approximately covers BA10—simply to emphasize the fact that BA10 does not include all parts of the prefrontal cortex.

Brodmann area 40 (BA40) is part of the parietal cortex in the human brain. The inferior part of BA40 is in the area of the supramarginal gyrus, which lies at the posterior end of the lateral fissure, in the inferior lateral part of the parietal lobe.

The lobes of the brain are the major identifiable zones of the cerebral cortex, and they comprise the surface of each hemisphere of the cerebrum. The two hemispheres, which are only roughly symmetrical in structure, are today considered as having six lobes each. The lobes are large areas that are anatomically distinguishable, and are also functionally distinct to some degree. Each lobe of the brain has numerous ridges, or gyri, and furrows, the sulci that constitute further subzones of the cortex. The expression "lobes of the brain" usually refers only to those of the cerebrum, not to the distinct areas of the cerebellum.

The inferior temporal gyrus is one of three gyri of the temporal lobe and is located below the middle temporal gyrus, connected behind with the inferior occipital gyrus; it also extends around the infero-lateral border on to the inferior surface of the temporal lobe, where it is limited by the inferior sulcus. This region is one of the higher levels of the ventral stream of visual processing, associated with the representation of objects, places, faces, and colors. It may also be involved in face perception, and in the recognition of numbers.

The parieto-occipital sulcus is a deep sulcus in the cerebral cortex that marks the boundary between the cuneus and precuneus, and also between the parietal and occipital lobes. Only a small part can be seen on the lateral surface of the hemisphere, its chief part being on the medial surface.

In neuroanatomy, a sulcus is a depression or groove in the cerebral cortex. It surrounds a gyrus, creating the characteristic folded appearance of the brain in humans and other mammals. The larger sulci are usually called fissures.

The superior longitudinal fasciculus (SLF) is an association tract in the brain that is composed of three separate components. It is present in both hemispheres and can be found lateral to the centrum semiovale and connects the frontal, occipital, parietal, and temporal lobes. This bundle of tracts (fasciculus) passes from the frontal lobe through the operculum to the posterior end of the lateral sulcus where they either radiate to and synapse on neurons in the occipital lobe, or turn downward and forward around the putamen and then radiate to and synapse on neurons in anterior portions of the temporal lobe.
Ralph Leslie Holloway, Jr. is a physical anthropologist at Columbia University and research associate with the American Museum of Natural History. Since obtaining his Ph.D from the University of California, Berkeley in 1964, Holloway has served as a professor of anthropology at Columbia. Holloway's interests lie in craniology, producing endocasts, primate behavior, biology of gender, sexual dimorphism in the corpus callosum, and other topics.

Dean Falk is an American academic neuroanthropologist who specializes in the evolution of the brain and cognition in higher primates. She is the Hale G. Smith Professor of Anthropology and a Distinguished Research Professor at Florida State University.
The Riddoch syndrome is a term coined by Zeki and Ffytche (1998) in a paper published in Brain. The term acknowledges the work of George Riddoch who was the first to describe a condition in which a form of visual impairment, caused by lesions in the occipital lobe, leaves the sufferer blind but able to distinguish visual stimuli with specific characteristics when these appear in the patient's blind field. The most common stimuli that can be perceived consciously are the presence and direction of fast moving objects ; in his work these moving objects were described as "vague and shadowy". Riddoch concluded from his observations that "movement may be recognized as a special visual perception".
The principles that govern the evolution of brain structure are not well understood. Brain to body size scales allometrically. Small bodied mammals have relatively large brains compared to their bodies whereas large mammals have smaller brain to body ratios. If brain weight is plotted against body weight for primates, the regression line of the sample points can indicate the brain power of a primate species. Lemurs for example fall below this line which means that for a primate of equivalent size, we would expect a larger brain size. Humans lie well above the line indicating that humans are more encephalized than lemurs. In fact, humans are more encephalized than all other primates.
The neuroanatomy of memory encompasses a wide variety of anatomical structures in the brain.
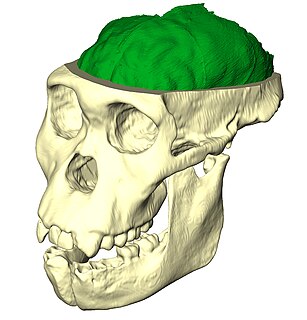
Paleoneurobiology is the study of brain evolution by analysis of brain endocasts to determine endocranial traits and volumes. Considered a subdivision of neuroscience, paleoneurobiology combines techniques from other fields of study including paleontology and archaeology. It reveals specific insight concerning human evolution. The cranium is unique in that it grows in response to the growth of brain tissue rather than genetic guidance, as is the case with bones that support movement. Fossil skulls and their endocasts can be compared to each other, to the skulls and fossils of recently deceased individuals, and even compared to those of other species to make inferences about functional anatomy, physiology and phylogeny. Paleoneurobiology is in large part influenced by developments in neuroscience as a whole; without substantial knowledge about current functionality, it would be impossible to make inferences about the functionality of ancient brains.

The occipital gyri (OcG) are three gyri in parallel, along the lateral portion of the occipital lobe, also referred to as a composite structure in the brain. The gyri are the superior occipital gyrus, the middle occipital gyrus, and the inferior occipital gyrus, and these are also known as the occipital face area. The superior and inferior occipital sulci separates the three occipital gyri.