Related Research Articles

The endometrium is the inner epithelial layer, along with its mucous membrane, of the mammalian uterus. It has a basal layer and a functional layer: the basal layer contains stem cells which regenerate the functional layer. The functional layer thickens and then is shed during menstruation in humans and some other mammals, including apes, Old World monkeys, some species of bat, the elephant shrew and the Cairo spiny mouse. In most other mammals, the endometrium is reabsorbed in the estrous cycle. During pregnancy, the glands and blood vessels in the endometrium further increase in size and number. Vascular spaces fuse and become interconnected, forming the placenta, which supplies oxygen and nutrition to the embryo and fetus. The speculated presence of an endometrial microbiota has been argued against.

In vitro fertilisation (IVF) is a process of fertilisation where an egg is combined with sperm in vitro. The process involves monitoring and stimulating a woman's ovulatory process, removing an ovum or ova from her ovaries and letting sperm fertilise them in a culture medium in a laboratory. After the fertilised egg (zygote) undergoes embryo culture for 2–6 days, it is transferred by catheter into the uterus, with the intention of establishing a successful pregnancy.

The menstrual cycle is a series of natural changes in hormone production and the structures of the uterus and ovaries of the female reproductive system that makes pregnancy possible. The ovarian cycle controls the production and release of eggs and the cyclic release of estrogen and progesterone. The uterine cycle governs the preparation and maintenance of the lining of the uterus (womb) to receive an embryo. These cycles are concurrent and coordinated, normally last between 21 and 35 days, with a median length of 28 days, and continue for about 30–45 years.

Artificial insemination is the deliberate introduction of sperm into a female's cervix or uterine cavity for the purpose of achieving a pregnancy through in vivo fertilization by means other than sexual intercourse. It is a fertility treatment for humans, and is a common practice in animal breeding, including dairy cattle and pigs.
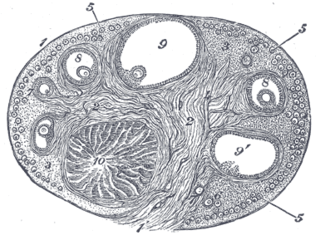
The corpus luteum is a temporary endocrine structure in female ovaries involved in the production of relatively high levels of progesterone, and moderate levels of estradiol, and inhibin A. It is the remains of the ovarian follicle that has released a mature ovum during a previous ovulation.
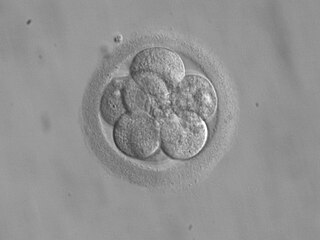
Embryo transfer refers to a step in the process of assisted reproduction in which embryos are placed into the uterus of a female with the intent to establish a pregnancy. This technique (which is often used in connection with in vitro fertilization, may be used in humans or in animals, in which situations the goals may vary.
Ovarian hyperstimulation syndrome (OHSS) is a medical condition that can occur in some women who take fertility medication to stimulate egg growth, and in other women in very rare cases. Most cases are mild, but rarely the condition is severe and can lead to serious illness or death.
The estrous cycle is a set of recurring physiological changes induced by reproductive hormones in females of mammalian subclass Theria. Estrous cycles start after sexual maturity in females and are interrupted by anestrous phases, otherwise known as "rest" phases, or by pregnancies. Typically, estrous cycles repeat until death. These cycles are widely variable in duration and frequency depending on the species. Some animals may display bloody vaginal discharge, often mistaken for menstruation. Many mammals used in commercial agriculture, such as cattle and sheep, may have their estrous cycles artificially controlled with hormonal medications for optimum productivity. The male equivalent, seen primarily in ruminants, is called rut.

In biology, folliculogenesis is the maturation of the ovarian follicle, a densely packed shell of somatic cells that contains an immature oocyte. Folliculogenesis describes the progression of a number of small primordial follicles into large preovulatory follicles that occurs in part during the menstrual cycle.

The menstrual cycle is on average 28 days in length. It begins with menses during the follicular phase and followed by ovulation and ending with the luteal phase. Unlike the follicular phase which can vary in length among individuals, the luteal phase is typically fixed at approximately 14 days and is characterized by changes to hormone levels, such as an increase in progesterone and estrogen levels, decrease in gonadotropins such as follicle-stimulating hormone (FSH) and luteinizing hormone (LH), changes to the endometrial lining to promote implantation of the fertilized egg, and development of the corpus luteum. In the absence of fertilization by sperm, the corpus luteum atrophies leading to a decrease in progesterone and estrogen, an increase in FSH and LH, and shedding of the endometrial lining (menses) to begin the menstrual cycle again.
Ovulation induction is the stimulation of ovulation by medication. It is usually used in the sense of stimulation of the development of ovarian follicles to reverse anovulation or oligoovulation.
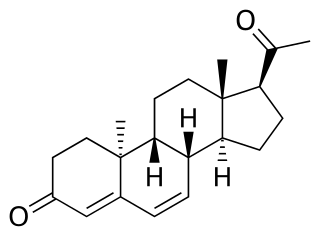
Dydrogesterone, sold under the brand name Duphaston among others, is a progestin medication which is used for a variety of indications, including threatened or recurrent miscarriage during pregnancy, dysfunctional bleeding, infertility due to luteal insufficiency, dysmenorrhea, endometriosis, secondary amenorrhea, irregular cycles, premenstrual syndrome, and as a component of menopausal hormone therapy. It is taken by mouth.

Oocyte cryopreservation is a procedure to preserve a woman's eggs (oocytes). This technique has been used to enable women to postpone pregnancy to a later date – whether for medical or social reasons. Several studies have shown that most infertility problems are due to germ cell deterioration related to aging. The intention of the procedure is that the woman may choose to have the eggs thawed, fertilized, and transferred to the uterus as embryos to facilitate a pregnancy in the future. The procedure's success rate varies depending on the age of the woman, with odds being higher in younger, adult women.
Controlled ovarian hyperstimulation is a technique used in assisted reproduction involving the use of fertility medications to induce ovulation by multiple ovarian follicles. These multiple follicles can be taken out by oocyte retrieval for use in in vitro fertilisation (IVF), or be given time to ovulate, resulting in superovulation which is the ovulation of a larger-than-normal number of eggs, generally in the sense of at least two. When ovulated follicles are fertilised in vivo, whether by natural or artificial insemination, there is a very high risk of a multiple pregnancy.

In vitro maturation (IVM) is the technique of letting the contents of ovarian follicles and the oocytes inside mature in vitro. It can be offered to women with infertility problems, combined with In Vitro Fertilization (IVF), offering women pregnancy without ovarian stimulation.
Poor ovarian reserve is a condition of low fertility characterized by 1): low numbers of remaining oocytes in the ovaries or 2) possibly impaired preantral oocyte development or recruitment. Recent research suggests that premature ovarian aging and premature ovarian failure may represent a continuum of premature ovarian senescence. It is usually accompanied by high FSH levels.
Transvaginal oocyte retrieval (TVOR), also referred to as oocyte retrieval (OCR), is a technique used in in vitro fertilization (IVF) in order to remove oocytes from the ovary of a woman, enabling fertilization outside the body. Transvaginal oocyte retrieval is more properly referred to as transvaginal ovum retrieval when the oocytes have matured into ova, as is normally the case in IVF. It can be also performed for egg donation, oocyte cryopreservation and other assisted reproduction technology such as ICSI.
Natural Cycle In Vitro Fertilization (IVF) is an assisted reproductive technique designed to closely mimic a woman's natural menstrual cycle. In traditional IVF, a woman's ovaries are stimulated with fertility medications to produce multiple eggs, which are then retrieved and fertilized outside the body. A natural cycle IVF, on the other hand, works with the woman's natural hormonal fluctuations and ovulation cycle.
Infertility in polycystic ovary disease (PCOS) is a hormonal imbalance in women that is thought to be one of the leading causes of female infertility. Polycystic ovary syndrome causes more than 75% of cases of anovulatory infertility.
Induction of final maturation of oocytes is a procedure that is usually performed as part of controlled ovarian hyperstimulation to render the oocytes fully developed and thereby resulting in optimal pregnancy chances. It is basically a replacement for the luteinizing hormone (LH) surge whose effects include final maturation in natural menstrual cycles.
References
- 1 2 3 4 5 6 7 8 Farquhar, Cindy; Marjoribanks, Jane (2018). "Assisted reproductive technology: an overview of Cochrane Reviews". Cochrane Database of Systematic Reviews . 2018 (8): CD010537. doi:10.1002/14651858.CD010537.pub5. ISSN 1465-1858. PMC 6953328 . PMID 30117155.
- 1 2 Van Der Linden, M.; Buckingham, K.; Farquhar, C.; Kremer, J. A. M.; Metwally, M. (2012). "Luteal phase support in assisted reproduction cycles". Human Reproduction Update. 18 (5): 473. doi: 10.1093/humupd/dms017 .
- ↑ Kyrou, D.; Kolibianakis, E. M.; Fatemi, H. M.; Tarlatzi, T. B.; Devroey, P.; Tarlatzis, B. C. (2011). "Increased live birth rates with GnRH agonist addition for luteal support in ICSI/IVF cycles: A systematic review and meta-analysis". Human Reproduction Update. 17 (6): 734–740. doi: 10.1093/humupd/dmr029 . PMID 21733980.
- 1 2 Barbosa, Marina Wanderley Paes; Valadares, Natália Paes Barbosa; Barbosa, Antônio César Paes; Amaral, Adelino Silva; Iglesias, José Rubens; Nastri, Carolina Oliveira; Martins, Wellington de Paula; Nakagawa, Hitomi Miura (2018). "Oral dydrogesterone vs. vaginal progesterone capsules for luteal-phase support in women undergoing embryo transfer: a systematic review and meta-analysis". JBRA Assisted Reproduction. 22 (2): 148–156. doi:10.5935/1518-0557.20180018. ISSN 1518-0557. PMC 5982562 . PMID 29488367.
- ↑ Rashidi, Batool Hossein; Ghazizadeh, Mahya; Tehrani Nejad, Ensieh Shahrokh; Bagheri, Maryam; Gorginzadeh, Mansoureh (2016). "Oral dydrogesterone for luteal support in frozen-thawed embryo transfer artificial cycles: A pilot randomized controlled trial". Asian Pacific Journal of Reproduction. 5 (6): 490–494. doi: 10.1016/j.apjr.2016.10.002 . ISSN 2305-0500.
- 1 2 Janelle Luk, MD; Pasquale Patrizio. "Luteal Phase Progesterone Support in ART/IVF". Medscape. Retrieved 2020-01-14.
- 1 2 Wiweko, Budi (2016). "Luteal Phase Support in Controlled Ovarian Hyperstimulation". Ovarian Stimulation Protocols. Springer. pp. 135–144. doi:10.1007/978-81-322-1121-1_11. ISBN 978-81-322-1120-4.
- ↑ Griesinger, Georg; Blockeel, Christophe; T. Sukhikh, Gennady; Patki, Ameet; Dhorepatil, Bharati; Yang, Dong-Zi; Chen, Zi-Jiang; Kahler, Elke; Pexman-Fieth, Claire; Tournaye, Herman (2018). "Oral dydrogesterone versus intravaginal micronized progesterone gel for luteal phase support in IVF: a randomized clinical trial". Human Reproduction. 33 (12): 2212–2221. doi: 10.1093/humrep/dey306 . ISSN 0268-1161. PMC 6238366 . PMID 30304457.
- ↑ Stadtmauer, Laurel; Waud, Kay (2014). "Progesterone Vaginal Ring for Luteal Support". The Journal of Obstetrics and Gynecology of India. 65 (1): 5–10. doi:10.1007/s13224-014-0634-0. ISSN 0971-9202. PMC 4342373 . PMID 25737615.
- ↑ Connell MT, Szatkowski JM, Terry N, DeCherney AH, Propst AM, Hill MJ (2015). "Timing luteal support in assisted reproductive technology: a systematic review". Fertil Steril. 103 (4): 939–946.e3. doi:10.1016/j.fertnstert.2014.12.125. PMC 4385437 . PMID 25638420.
- ↑ Green, Katherine A.; Zolton, Jessica R.; Schermerhorn, Sophia M.V.; Lewis, Terrence D.; Healy, Mae W.; Terry, Nancy; DeCherney, Alan H.; Hill, Micah J. (2017). "Progesterone luteal support after ovulation induction and intrauterine insemination: an updated systematic review and meta-analysis". Fertility and Sterility. 107 (4): 924–933.e5. doi: 10.1016/j.fertnstert.2017.01.011 . ISSN 0015-0282. PMID 28238492.
- ↑ Lien, Y.R.; Jou, G.; Yang, P.; Chen, S. (2015). "The duration of luteal phase support by progesterone in fresh transfer cycles can be determined by corpus luteum rescue or not". Fertility and Sterility. 104 (3): e344–e345. doi: 10.1016/j.fertnstert.2015.07.1074 . ISSN 0015-0282.