The lanthanide or lanthanoid series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttrium, are often collectively known as the rare-earth elements or rare-earth metals.

In chemistry, the term transition metal has three possible definitions:

Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer (passivation) somewhat stabilizes the free metal against further oxidation.

Group 5 is a group of elements in the periodic table. Group 5 contains vanadium (V), niobium (Nb), tantalum (Ta) and dubnium (Db). This group lies in the d-block of the periodic table. This group is sometimes called the vanadium group or vanadium family after its lightest member; however, the group itself has not acquired a trivial name because it belongs to the broader grouping of the transition metals.
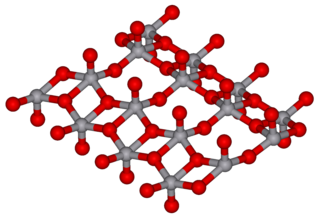
Vanadium(V) oxide (vanadia) is the inorganic compound with the formula V2O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst.

Heusler compounds are magnetic intermetallics with face-centered cubic crystal structure and a composition of XYZ (half-Heuslers) or X2YZ (full-Heuslers), where X and Y are transition metals and Z is in the p-block. The term derives from the name of German mining engineer and chemist Friedrich Heusler, who studied such a compound (Cu2MnAl) in 1903. Many of these compounds exhibit properties relevant to spintronics, such as magnetoresistance, variations of the Hall effect, ferro-, antiferro-, and ferrimagnetism, half- and semimetallicity, semiconductivity with spin filter ability, superconductivity, topological band structure and are actively studied as Thermoelectric materials. Their magnetism results from a double-exchange mechanism between neighboring magnetic ions. Manganese, which sits at the body centers of the cubic structure, was the magnetic ion in the first Heusler compound discovered. (See the Bethe–Slater curve for details of why this happens.)

Lutetium(III) oxide, a white solid, is a cubic compound of lutetium sometimes used in the preparation of specialty glasses. It is also called lutecia. It is a lanthanide oxide, also known as a rare earth.
Ytterbium-doped Lutetium orthovanadate, typically abbreviated Yb:LuVO4, is an active laser medium. The peak absorption cross section for the pi-polarization is 8.42×10−20 cm² at 985 nm, and the stimulated emission cross section at 1020 nm is 1.03×10−20 cm².

In chemistry, a vanadate is an anionic coordination complex of vanadium. Often vanadate refers to oxoanions of vanadium, most of which exist in its highest oxidation state of +5. The complexes [V(CN)6]3− and [V2Cl9]3− are referred to as hexacyanovanadate(III) and nonachlorodivanadate(III), respectively.

Mercury selenide is a chemical compound of mercury and selenium. It is a grey-black crystalline solid semi-metal with a sphalerite structure. The lattice constant is 0.608 nm.

Ammonium metavanadate is the inorganic compound with the formula NH4VO3. It is a white salt, although samples are often yellow owing to impurities of V2O5. It is an important intermediate in the purification of vanadium.
Lutetium aluminum garnet (commonly abbreviated LuAG, molecular formula Lu3Al5O12) is an inorganic compound with a unique crystal structure primarily known for its use in high-efficiency laser devices. LuAG is also useful in the synthesis of transparent ceramics.

Helimagnetism is a form of magnetic ordering where spins of neighbouring magnetic moments arrange themselves in a spiral or helical pattern, with a characteristic turn angle of somewhere between 0 and 180 degrees. It results from the competition between ferromagnetic and antiferromagnetic exchange interactions. It is possible to view ferromagnetism and antiferromagnetism as helimagnetic structures with characteristic turn angles of 0 and 180 degrees respectively. Helimagnetic order breaks spatial inversion symmetry, as it can be either left-handed or right-handed in nature.
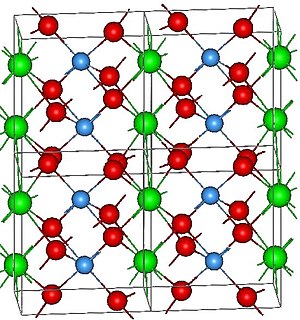
Lutetium tantalate is a chemical compound of lutetium, tantalum and oxygen with the formula LuTaO4. With a density of 9.81 g/cm3, this salt is the densest known white stable material. (Although thorium dioxide ThO2 is also white and has a higher density of 10 g/cm3, it is radioactively unstable; while not radioactive enough to make it unstable as a material, even its low rate of decay is still too much for certain uses such as phosphors for detecting ionising radiation.) The white color and high density of LuTaO4 make it ideal for phosphor applications, though the high cost of lutetium is a hindrance.
Langbeinites are a family of crystalline substances based on the structure of langbeinite with general formula M2M'2(SO4)3, where M is a large univalent cation, and M' is a small divalent cation. The sulfate group, SO2−4, can be substituted by other tetrahedral anions with a double negative charge such as tetrafluoroberyllate, selenate, chromate, molybdate, or tungstates. Although monofluorophosphates are predicted, they have not been described. By redistributing charges other anions with the same shape such as phosphate also form langbeinite structures. In these the M' atom must have a greater charge to balance the extra three negative charges.
CeCoIn5 ("Cerium-Cobalt-Indium 5") is a heavy-fermion superconductor with a layered crystal structure, with somewhat two-dimensional electronic transport properties. The critical temperature of 2.3 K is the highest among all of the Ce-based heavy-fermion superconductors.

Iron boride refers to various inorganic compounds with the formula FexBy. Two main iron borides are FeB and Fe2B. Some iron borides possess useful properties such as magnetism, electrical conductivity, corrosion resistance and extreme hardness. Some iron borides have found use as hardening coatings for iron. Iron borides have properties of ceramics such as high hardness, and properties of metal properties, such as thermal conductivity and electrical conductivity. Boride coatings on iron are superior mechanical, frictional, and anti-corrosive. Iron monoboride (FeB) is a grey powder that is insoluble in water. FeB is harder than Fe2B, but is more brittle and more easily fractured upon impact.
Oxyphosphides are chemical compounds formally containing the group PO, with one phosphorus and one oxygen atom. The phosphorus and oxygen are not bound together as in phosphates or phosphine oxides, instead they are bound separately to the cations (metals), and could be considered as a mixed phosphide-oxide compound. So a compound with OmPn requires cations to balance a negative charge of 2m+3n. The cations will have charges of +2 or +3. The trications are often rare earth elements or actinides. They are in the category of oxy-pnictide compounds.
Manganese monosilicide (MnSi) is an intermetallic compound, a silicide of manganese. It occurs in cosmic dust as the mineral brownleeite. MnSi has a cubic crystal lattice with no inversion center; therefore its crystal structure is helical, with right-hand and left-hand chiralities.
Corundum is the name for a structure prototype in inorganic solids, derived from the namesake polymorph of aluminum oxide (α-Al2O3). Other compounds, especially among the inorganic solids, exist in corundum structure, either in ambient or other conditions. Corundum structures are associated with metal-insulator transition, ferroelectricity, polar magnetism, and magnetoelectric effects.












