Related Research Articles

A DNA vaccine is a type of vaccine that transfects a specific antigen-coding DNA sequence into the cells of an organism as a mechanism to induce an immune response.

Squalene is an organic compound. It is a triterpenoid with the formula C30H50. It is a colourless oil, although impure samples appear yellow. It was originally obtained from shark liver oil (hence its name, as Squalus is a genus of sharks). All plants and animals produce squalene as a biochemical intermediate to sterol biosynthesis. An estimated 12% of bodily squalene in humans is found in sebum. Squalene has a role in topical skin lubrication and protection.

Influenza vaccines, also known as flu shots, are vaccines that protect against infection by influenza viruses. New versions of the vaccines are developed twice a year, as the influenza virus rapidly changes. While their effectiveness varies from year to year, most provide modest to high protection against influenza. The United States Centers for Disease Control and Prevention (CDC) estimates that vaccination against influenza reduces sickness, medical visits, hospitalizations, and deaths. Immunized workers who do catch the flu return to work half a day sooner on average. Vaccine effectiveness in those over 65 years old remains uncertain due to a lack of high-quality research. Vaccinating children may protect those around them.
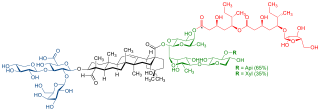
QS-21 is a purified plant extract used as a vaccine adjuvant. It is derived from the soap bark tree, which is native to the countries of Chile, Peru, and Bolivia. The crude drug is imported from Peru and Chile.
Virus-like particles (VLPs) are molecules that closely resemble viruses, but are non-infectious because they contain no viral genetic material. They can be naturally occurring or synthesized through the individual expression of viral structural proteins, which can then self assemble into the virus-like structure. Combinations of structural capsid proteins from different viruses can be used to create recombinant VLPs. Both in-vivo assembly and in-vitro assembly have been successfully shown to form virus-like particles. VLPs derived from the Hepatitis B virus (HBV) and composed of the small HBV derived surface antigen (HBsAg) were described in 1968 from patient sera. VLPs have been produced from components of a wide variety of virus families including Parvoviridae, Retroviridae, Flaviviridae, Paramyxoviridae and bacteriophages. VLPs can be produced in multiple cell culture systems including bacteria, mammalian cell lines, insect cell lines, yeast and plant cells.

Rino Rappuoli is head of vaccine research and development (R&D) at GlaxoSmithKline (GSK) Vaccines. Previously, he has served as visiting scientist at Rockefeller University and Harvard Medical School and held roles at Sclavo, Vaccine Research and CSO, Chiron Corporation, and Novartis Vaccines.
In immunology, an adjuvant is a substance that increases or modulates the immune response to a vaccine. The word "adjuvant" comes from the Latin word adiuvare, meaning to help or aid. "An immunologic adjuvant is defined as any substance that acts to accelerate, prolong, or enhance antigen-specific immune responses when used in combination with specific vaccine antigens."

A malaria vaccine is a vaccine that is used to prevent malaria. The only approved malaria vaccine is RTS,S, known by the brand name Mosquirix. As of April 2022, the vaccine has been given to 1 million children living in areas with moderate-to-high malaria transmission. It requires at least three doses in infants by age 2, with a fourth dose extending the protection for another 1-2 years. The vaccine reduces hospital admissions from severe malaria by around 30%.

Pandemrix is an influenza vaccine for influenza pandemics, such as the 2009 flu pandemic. The vaccine was developed by GlaxoSmithKline (GSK) and patented in September 2006.
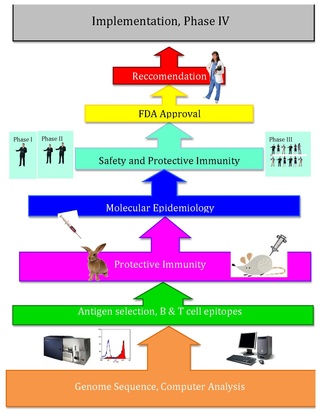
Reverse vaccinology is an improvement of vaccinology that employs bioinformatics and reverse pharmacology practices, pioneered by Rino Rappuoli and first used against Serogroup B meningococcus. Since then, it has been used on several other bacterial vaccines.

An inactivated vaccine is a vaccine consisting of virus particles, bacteria, or other pathogens that have been grown in culture and then killed to destroy disease-producing capacity. In contrast, live vaccines use pathogens that are still alive. Pathogens for inactivated vaccines are grown under controlled conditions and are killed as a means to reduce infectivity and thus prevent infection from the vaccine.

The 2009 swine flu pandemic vaccines were influenza vaccines developed to protect against the pandemic H1N1/09 virus. These vaccines either contained inactivated (killed) influenza virus, or weakened live virus that could not cause influenza. The killed virus was injected, while the live virus was given as a nasal spray. Both these types of vaccine were produced by growing the virus in chicken eggs. Around three billion doses were produced, with delivery in November 2009.
AS03 is the trade name for a squalene-based immunologic adjuvant used in various vaccine products by GlaxoSmithKline (GSK). It is used, for example, in GSK's A/H1N1 pandemic flu vaccine Pandemrix. It is also in Arepanrix and the Q-pan for H5N1 influenza.A dose of AS03 adjuvant contains

NeuVax is a peptide vaccine aimed at preventing or delaying the recurrence of breast cancer in cancer survivors who achieve remission after standard of care treatment. The product's developer is the US biotechnology company Galena Biopharma.

A H5N1 vaccine is an influenza vaccine intended to provide immunization to influenza A virus subtype H5N1.
Cell-based vaccines are developed from mammalian or more rarely avian or insect cell lines rather than the more common method which uses the cells in embryonic chicken eggs to develop the antigens. The potential use of cell culture techniques in developing viral vaccines has been widely investigated in the 2000s as a complementary and alternative platform to the current egg-based strategies.
CRM197 is a non-toxic mutant of diphtheria toxin, currently used as a carrier protein for polysaccharides and haptens to make them immunogenic. There is some dispute about the toxicity of CRM197, with evidence that it is toxic to yeast cells and some mammalian cell lines.
Samira Mubareka is a Canadian microbiologist who is a clinical scientist at the Sunnybrook Health Sciences Centre in Toronto, Ontario. Her research considers the influenza virus, viral transmission and aerobiology. During the COVID-19 pandemic Mubareka isolated the genome of Severe acute respiratory syndrome coronavirus 2 in an effort to improve detection and diagnostics. She served as a member of the Ontario COVID-19 Science Advisory Table.

Recombinant subunit vaccines are biological preparations that are composed of microbial subunits produced using recombinant DNA technology. They act to provide active acquired immunity to infectious diseases. The first recombinant subunit vaccine was produced in the mid-1980s to protect people from Hepatitis B. Notable recombinant subunit vaccines licensed include ENGERIX-B, GARDASIL-9, FLUBLOK(influenza), SHINGRIX and NUVAXOVID.
References
- ↑ O’Hagan, Derek T; Ott, Gary S; Nest, Gary Van; Rappuoli, Rino; Giudice, Giuseppe Del (2014). "The history of MF59® adjuvant: a phoenix that arose from the ashes". Expert Review of Vaccines. 12 (1): 13–30. doi:10.1586/erv.12.140. PMID 23256736.
- ↑ O’Hagan, Derek T; Rappuoli, Rino; De Gregorio, Ennio; Tsai, Theodore; Del Giudice, Giuseppe (2014). "MF59 adjuvant: the best insurance against influenza strain diversity". Expert Review of Vaccines. 10 (4): 447–462. doi:10.1586/erv.11.23. PMID 21506643.
- ↑ Trivedi, B. (2006). "Profile of Rino Rappuoli". Proceedings of the National Academy of Sciences. 103 (29): 10831–10833. Bibcode:2006PNAS..10310831T. doi:10.1073/pnas.0604892103. PMC 1544134 . PMID 16832044.
- ↑ "CDC Flu Vaccine With Adjuvant". Archived from the original on 2017-08-21. Retrieved 2018-10-11.
- ↑ MF59 Adjuvant Fact Sheet Archived 2014-03-09 at the Wayback Machine