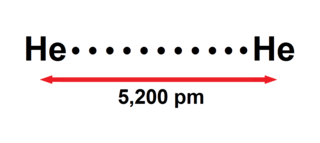In condensed matter physics, a Bose–Einstein condensate (BEC) is a state of matter that is typically formed when a gas of bosons at very low densities is cooled to temperatures very close to absolute zero. Under such conditions, a large fraction of bosons occupy the lowest quantum state, at which microscopic quantum-mechanical phenomena, particularly wavefunction interference, become apparent macroscopically. More generally, condensation refers to the appearance of macroscopic occupation of one or several states: for example, in BCS theory, a superconductor is a condensate of Cooper pairs. As such, condensation can be associated with phase transition, and the macroscopic occupation of the state is the order parameter.

A crystallographic defect is an interruption of the regular patterns of arrangement of atoms or molecules in crystalline solids. The positions and orientations of particles, which are repeating at fixed distances determined by the unit cell parameters in crystals, exhibit a periodic crystal structure, but this is usually imperfect. Several types of defects are often characterized: point defects, line defects, planar defects, bulk defects. Topological homotopy establishes a mathematical method of characterization.

In quantum mechanics, the Pauli exclusion principle states that two or more identical particles with half-integer spins cannot simultaneously occupy the same quantum state within a system that obeys the laws of quantum mechanics. This principle was formulated by Austrian physicist Wolfgang Pauli in 1925 for electrons, and later extended to all fermions with his spin–statistics theorem of 1940.

In nuclear physics, the island of stability is a predicted set of isotopes of superheavy elements that may have considerably longer half-lives than known isotopes of these elements. It is predicted to appear as an "island" in the chart of nuclides, separated from known stable and long-lived primordial radionuclides. Its theoretical existence is attributed to stabilizing effects of predicted "magic numbers" of protons and neutrons in the superheavy mass region.
An extended periodic table theorizes about chemical elements beyond those currently known and proven. The element with the highest atomic number known is oganesson (Z = 118), which completes the seventh period (row) in the periodic table. All elements in the eighth period and beyond thus remain purely hypothetical.
Flerovium is a superheavy synthetic chemical element; it has symbol Fl and atomic number 114. It is an extremely radioactive synthetic element, named after the Flerov Laboratory of Nuclear Reactions of the Joint Institute for Nuclear Research in Dubna, Russia, where the element was discovered in 1999. The lab's name, in turn, honours Russian physicist Georgy Flyorov. IUPAC adopted the name on 30 May 2012. The name and symbol had previously been proposed for element 102 (nobelium), but was not accepted by IUPAC at that time.

In nuclear physics, a magic number is a number of nucleons such that they are arranged into complete shells within the atomic nucleus. As a result, atomic nuclei with a 'magic' number of protons or neutrons are much more stable than other nuclei. The seven most widely recognized magic numbers as of 2019 are 2, 8, 20, 28, 50, 82, and 126.
A Rydberg atom is an excited atom with one or more electrons that have a very high principal quantum number, n. The higher the value of n, the farther the electron is from the nucleus, on average. Rydberg atoms have a number of peculiar properties including an exaggerated response to electric and magnetic fields, long decay periods and electron wavefunctions that approximate, under some conditions, classical orbits of electrons about the nuclei. The core electrons shield the outer electron from the electric field of the nucleus such that, from a distance, the electric potential looks identical to that experienced by the electron in a hydrogen atom.
This is a timeline of subatomic particle discoveries, including all particles thus far discovered which appear to be elementary given the best available evidence. It also includes the discovery of composite particles and antiparticles that were of particular historical importance.
Darmstadtium (110Ds) is a synthetic element, and thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 269Ds in 1994. There are 11 known radioisotopes from 267Ds to 281Ds and 2 or 3 known isomers. The longest-lived isotope is 281Ds with a half-life of 14 seconds.
Flerovium (114Fl) is a synthetic element, and thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 289Fl in 1999. Flerovium has six known isotopes, along with the unconfirmed 290Fl, and possibly two nuclear isomers. The longest-lived isotope is 289Fl with a half-life of 1.9 seconds, but 290Fl may have a longer half-life of 19 seconds.
Livermorium (116Lv) is an artificial element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no stable isotopes. The first isotope to be synthesized was 293Lv in 2000. There are five known radioisotopes, with mass numbers 288 and 290–293, as well as a few suggestive indications of a possible heavier isotope 294Lv. The longest-lived known isotope is 293Lv with a half-life of 70 ms.
This page deals with the electron affinity as a property of isolated atoms or molecules. Solid state electron affinities are not listed here.

A nuclear clock or nuclear optical clock is a notional clock that would use the frequency of a nuclear transition as its reference frequency, in the same manner as an atomic clock uses the frequency of an electronic transition in an atom's shell. Such a clock is expected to be more accurate than the best current atomic clocks by a factor of about 10, with an achievable accuracy approaching the 10−19 level. The only nuclear state suitable for the development of a nuclear clock using existing technology is thorium-229m, a nuclear isomer of thorium-229 and the lowest-energy nuclear isomer known. With an energy of about 8 eV, the corresponding ground-state transition is expected to be in the vacuum ultraviolet wavelength region around 150 nm, which would make it accessible to laser excitation. A comprehensive review can be found in reference.
In atomic physics, a spin-destructioncollision is a physical impact where the spin angular momentum of an atom is irretrievably scrambled.

In condensed-matter physics, a collision cascade is a set of nearby adjacent energetic collisions of atoms induced by an energetic particle in a solid or liquid.
Interatomic Coulombic decay (ICD) is a general, fundamental property of atoms and molecules that have neighbors. Interatomic (intermolecular) Coulombic decay is a very efficient interatomic (intermolecular) relaxation process of an electronically excited atom or molecule embedded in an environment. Without the environment the process cannot take place. Until now it has been mainly demonstrated for atomic and molecular clusters, independently of whether they are of van-der-Waals or hydrogen bonded type.
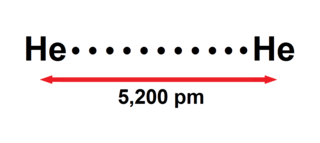
The helium dimer is a van der Waals molecule with formula He2 consisting of two helium atoms. This chemical is the largest diatomic molecule—a molecule consisting of two atoms bonded together. The bond that holds this dimer together is so weak that it will break if the molecule rotates, or vibrates too much. It can only exist at very low cryogenic temperatures.

David Tománek (born July 1954) is a U.S.-Swiss physicist of Czech origin and researcher in nanoscience and nanotechnology. He is Emeritus Professor of Physics at Michigan State University. He is known for predicting the structure and calculating properties of surfaces, atomic clusters including the C60 buckminsterfullerene, nanotubes, nanowires and nanohelices, graphene, and two-dimensional materials including phosphorene.
Christopher John Pethick is a British theoretical physicist, specializing in many-body theory, ultra-cold atomic gases, and the physics of neutron stars and stellar collapse.