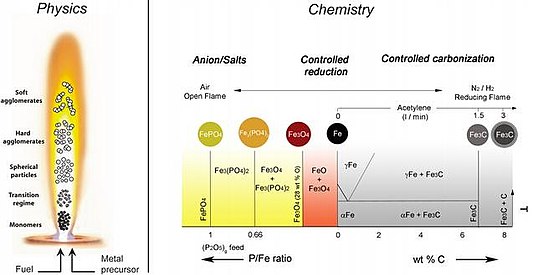
Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally as the mineral magnetite. As the mineral known as hematite, Fe2O3 is the main source of iron for the steel industry. Fe2O3 is readily attacked by acids. Iron(III) oxide is often called rust, since rust shares several properties and has a similar composition; however, in chemistry, rust is considered an ill-defined material, described as hydrous ferric oxide.

Iron oxides are chemical compounds composed of iron and oxygen. Several iron oxides are recognized. Often they are non-stoichiometric. Oxyhydroxides are a related class of compounds, perhaps the best known of which is rust.

Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With the exception of extremely rare native iron deposits, it is the most magnetic of all the naturally occurring minerals on Earth. Naturally magnetized pieces of magnetite, called lodestone, will attract small pieces of iron, which is how ancient peoples first discovered the property of magnetism.

Ferrofluid is a liquid that is attracted to the poles of a magnet. It is a colloidal liquid made of nanoscale ferromagnetic or ferrimagnetic particles suspended in a carrier fluid. Each magnetic particle is thoroughly coated with a surfactant to inhibit clumping. Large ferromagnetic particles can be ripped out of the homogeneous colloidal mixture, forming a separate clump of magnetic dust when exposed to strong magnetic fields. The magnetic attraction of tiny nanoparticles is weak enough that the surfactant's Van der Waals force is sufficient to prevent magnetic clumping or agglomeration. Ferrofluids usually do not retain magnetization in the absence of an externally applied field and thus are often classified as "superparamagnets" rather than ferromagnets.

Maghemite (Fe2O3, γ-Fe2O3) is a member of the family of iron oxides. It has the same formula as hematite, but the same spinel ferrite structure as magnetite (Fe3O4) and is also ferrimagnetic. It is sometimes spelled as "maghaemite".

A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At the lowest range, metal particles smaller than 1 nm are usually called atom clusters instead.

Iron(II,III) oxide, or black iron oxide, is the chemical compound with formula Fe3O4. It occurs in nature as the mineral magnetite. It is one of a number of iron oxides, the others being iron(II) oxide (FeO), which is rare, and iron(III) oxide (Fe2O3) which also occurs naturally as the mineral hematite. It contains both Fe2+ and Fe3+ ions and is sometimes formulated as FeO ∙ Fe2O3. This iron oxide is encountered in the laboratory as a black powder. It exhibits permanent magnetism and is ferrimagnetic, but is sometimes incorrectly described as ferromagnetic. Its most extensive use is as a black pigment (see: Mars Black). For this purpose, it is synthesized rather than being extracted from the naturally occurring mineral as the particle size and shape can be varied by the method of production.
Magnetic particle imaging (MPI) is an emerging non-invasive tomographic technique that directly detects superparamagnetic nanoparticle tracers. The technology has potential applications in diagnostic imaging and material science. Currently, it is used in medical research to measure the 3-D location and concentration of nanoparticles. Imaging does not use ionizing radiation and can produce a signal at any depth within the body. MPI was first conceived in 2001 by scientists working at the Royal Philips Research lab in Hamburg. The first system was established and reported in 2005. Since then, the technology has been advanced by academic researchers at several universities around the world. The first commercial MPI scanners have recently become available from Magnetic Insight and Bruker Biospin.

A ferrite is a ceramic material made by mixing and firing iron(III) oxide with one or more additional metallic elements, such as strontium, barium, manganese, nickel, and zinc. They are ferrimagnetic, meaning they are attracted by magnetic fields and can be magnetized to become permanent magnets. Unlike other ferromagnetic materials, most ferrites are not electrically conductive, making them useful in applications like magnetic cores for transformers to suppress eddy currents. Ferrites can be divided into two families based on their resistance to being demagnetized.

Nanochemistry is an emerging sub-discipline of the chemical and material sciences that deals with the development of new methods for creating nanoscale materials. The term "nanochemistry" was first used by Ozin in 1992 as 'the uses of chemical synthesis to reproducibly afford nanomaterials from the atom "up", contrary to the nanoengineering and nanophysics approach that operates from the bulk "down"'. Nanochemistry focuses on solid-state chemistry that emphasizes synthesis of building blocks that are dependent on size, surface, shape, and defect properties, rather than the actual production of matter. Atomic and molecular properties mainly deal with the degrees of freedom of atoms in the periodic table. However, nanochemistry introduced other degrees of freedom that controls material's behaviors by transformation into solutions. Nanoscale objects exhibit novel material properties, largely as a consequence of their finite small size. Several chemical modifications on nanometer-scaled structures approve size dependent effects.
Magnetofection is a transfection method that uses magnetic fields to concentrate particles containing vectors to target cells in the body. Magnetofection has been adapted to a variety of vectors, including nucleic acids, non-viral transfection systems, and viruses. This method offers advantages such as high transfection efficiency and biocompatibility which are balanced with limitations.
Magnetic-targeted carriers, also known as MTCs or magnetic vehicles, are micro- or nanoparticles that carry an anticancer drug to the target site by using an external magnetic field and field gradient to direct the desired drug. Usually, the complex involves microscopic beads of activated carbon, which bind the anticancer drug. A magnet applied from outside the body then can direct the drug to the tumor site. This can keep a larger dose of the drug at the tumor site for a longer period of time, and help protect healthy tissue from the side effects of chemotherapy.
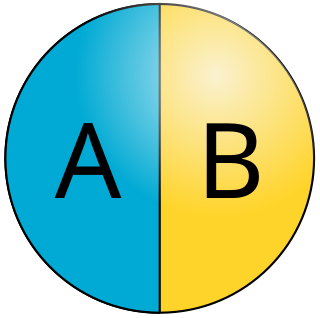
Janus particles are special types of nanoparticles or microparticles whose surfaces have two or more distinct physical properties. This unique surface of Janus particles allows two different types of chemistry to occur on the same particle. The simplest case of a Janus particle is achieved by dividing the particle into two distinct parts, each of them either made of a different material, or bearing different functional groups. For example, a Janus particle may have one half of its surface composed of hydrophilic groups and the other half hydrophobic groups, the particles might have two surfaces of different color, fluorescence, or magnetic properties. This gives these particles unique properties related to their asymmetric structure and/or functionalization.
Magnetic Nanorings are a form of magnetic nanoparticles, typically made of iron oxide in the shape of a ring. They have multiple applications in the medical field and computer engineering. In experimental trials, they provide a more localized form of cancer treatment by attacking individual cells instead of a general cancerous region of the body, as well as a clearer image of tumors by improving accuracy of cancer cell identification. They also allow for a more efficient and smaller, MRAM, which helps reduce the size of the technology houses it. Magnetic nanorings can be produced in various compositions, shapes, and sizes by using hematite nanorings as the base structure.

Iron oxide nanoparticles are iron oxide particles with diameters between about 1 and 100 nanometers. The two main forms are composed of magnetite and its oxidized form maghemite. They have attracted extensive interest due to their superparamagnetic properties and their potential applications in many fields including molecular imaging.
Cuprospinel is a mineral. Cuprospinel is an inverse spinel with the chemical formula CuFe2O4, where copper substitutes some of the iron cations in the structure. Its structure is similar to that of magnetite, Fe3O4, yet with slightly different chemical and physical properties due to the presence of copper.

In materials and electric battery research, cobalt oxide nanoparticles usually refers to particles of cobalt(II,III) oxide Co
3O
4 of nanometer size, with various shapes and crystal structures.

Iron–platinum nanoparticles are 3D superlattices composed of an approximately equal atomic ratio of Fe and Pt. Under standard conditions, FePt NPs exist in the face-centered cubic phase but can change to a chemically ordered face-centered tetragonal phase as a result of thermal annealing. Currently there are many synthetic methods such as water-in-oil microemulsion, one-step thermal synthesis with metal precursors, and exchanged-coupled assembly for making FePt NPs. An important property of FePt NPs is their superparamagnetic character below 10 nanometers. The superparamagnetism of FePt NPs has made them attractive candidates to be used as MRI/CT scanning agents and a high-density recording material.
Magnetoelastic filaments are one-dimensional composite structures that exhibit both magnetic and elastic properties. Interest in these materials tends to focus on the ability to precisely control mechanical events using an external magnetic field. Like piezoelectricity materials, they can be used as actuators, but do not need to be physically connected to a power source. The conformations adopted by magnetoelastic filaments are dictated by the competition between its elastic and magnetic properties.
Praseodymium(III,IV) oxide is the inorganic compound with the formula Pr6O11 that is insoluble in water. It has a cubic fluorite structure. It is the most stable form of praseodymium oxide at ambient temperature and pressure.