
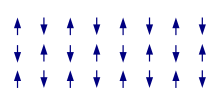

The term magnetic structure of a material pertains to the ordered arrangement of magnetic spins, typically within an ordered crystallographic lattice. Its study is a branch of solid-state physics.



The term magnetic structure of a material pertains to the ordered arrangement of magnetic spins, typically within an ordered crystallographic lattice. Its study is a branch of solid-state physics.
Most solid materials are non-magnetic, that is, they do not display a magnetic structure. Due to the Pauli exclusion principle, each state is occupied by electrons of opposing spins, so that the charge density is compensated everywhere and the spin degree of freedom is trivial. Still, such materials typically do show a weak magnetic behaviour, e.g. due to diamagnetism or Pauli paramagnetism.
The more interesting case is when the material's electron spontaneously break above-mentioned symmetry. For ferromagnetism in the ground state, there is a common spin quantization axis and a global excess of electrons of a given spin quantum number, there are more electrons pointing in one direction than in the other, giving a macroscopic magnetization (typically, the majority electrons are chosen to point up). In the most simple (collinear) cases of antiferromagnetism, there is still a common quantization axis, but the electronic spins are pointing alternatingly up and down, leading again to cancellation of the macroscopic magnetization. However, specifically in the case of frustration of the interactions, the resulting structures can become much more complicated, with inherently three-dimensional orientations of the local spins. Finally, ferrimagnetism as prototypically displayed by magnetite is in some sense an intermediate case: here the magnetization is globally uncompensated as in ferromagnetism, but the local magnetization points in different directions.
The above discussion pertains to the ground state structure. Of course, finite temperatures lead to excitations of the spin configuration. Here two extreme points of view can be contrasted: in the Stoner picture of magnetism (also called itinerant magnetism), the electronic states are delocalized, and their mean-field interaction leads to the symmetry breaking. In this view, with increasing temperature the local magnetization would thus decrease homogeneously, as single delocalized electrons are moved from the up- to the down-channel. On the other hand, in the local-moment case the electronic states are localized to specific atoms, giving atomic spins, which interact only over a short range and typically are analyzed with the Heisenberg model. Here, finite temperatures lead to a deviation of the atomic spins' orientations from the ideal configuration, thus for a ferromagnet also decreasing the macroscopic magnetization.
For localized magnetism, many magnetic structures can be described by magnetic space groups, which give a precise accounting for all possible symmetry groups of up/down configurations in a three-dimensional crystal. However, this formalism is unable to account for some more complex magnetic structures, such as those found in helimagnetism.
Such ordering can be studied by observing the magnetic susceptibility as a function of temperature and/or the size of the applied magnetic field, but a truly three-dimensional picture of the arrangement of the spins is best obtained by means of neutron diffraction. [1] [2] Neutrons are primarily scattered by the nuclei of the atoms in the structure. At a temperature above the ordering point of the magnetic moments, where the material behaves as a paramagnetic one, neutron diffraction will therefore give a picture of the crystallographic structure only. Below the ordering point, e.g. the Néel temperature of an antiferromagnet or the Curie-point of a ferromagnet the neutrons will also experience scattering from the magnetic moments because they themselves possess spin. The intensities of the Bragg reflections will therefore change. In fact in some cases entirely new Bragg-reflections will occur if the unit cell of the ordering is larger than that of the crystallographic structure. This is a form of superstructure formation. Thus the symmetry of the total structure may well differ from the crystallographic substructure. It needs to be described by one of the 1651 magnetic (Shubnikov) groups rather than one of the non-magnetic space groups. [3]
Although ordinary X-ray diffraction is 'blind' to the arrangement of the spins, it has become possible to use a special form of X-ray diffraction to study magnetic structure. If a wavelength is selected that is close to an absorption edge of one of elements contained in the materials the scattering becomes anomalous and this component to the scattering is (somewhat) sensitive to the non-spherical shape of the outer electrons of an atom with an unpaired spin. This means that this type of anomalous X-ray diffraction does contain information of the desired type.
More recently, table-top techniques are being developed which allow magnetic structures to be studied without recourse to neutron or synchrotron sources. [4]
Only three elements are ferromagnetic at room temperature and pressure: iron, cobalt, and nickel. This is because their Curie temperature, Tc, is higher than room temperature (Tc > 298K). Gadolinium has a spontaneous magnetization just below room temperature (293 K) and is sometimes counted as the fourth ferromagnetic element. There has been some suggestion that Gadolinium has helimagnetic ordering, [5] but others defend the longstanding view that Gadolinium is a conventional ferromagnet. [6]
The elements Dysprosium and Erbium each have two magnetic transitions. They are paramagnetic at room temperature, but become helimagnetic below their respective Néel temperatures, and then become ferromagnetic below their Curie temperatures. The elements Holmium, Terbium, and Thulium display even more complicated magnetic structures. [7]
There is also antiferromagnetic ordering, which becomes disordered above the Néel temperature. Chromium is somewhat like a simple antiferromagnet, but also has an incommensurate spin density wave modulation on top of the simple up-down spin alternation. [8] Manganese (in the α-Mn form) has 29 atoms unit cell, leading to a complex, but commensurate antiferromagnetic arrangement at low temperatures (magnetic space group P42'm'). [9] [10] Unlike most elements, which are magnetic due to electrons, the magnetic ordering of copper and silver is dominated by the much weaker nuclear magnetic moment, (compare Bohr magneton and nuclear magneton) leading to transition temperatures near absolute zero. [11] [12]
Those elements which become superconductors exhibit superdiamagnetism below a critical temperature.
| No. | Name | Superconducting Tc | Curie temperature | Néel temperature |
|---|---|---|---|---|
| 3 | Lithium | 0.0004 K [13] | ||
| 13 | Aluminum | 1.18 K [13] | ||
| 22 | Titanium | 0.5 K [13] | ||
| 23 | Vanadium | 5.4 K [13] | ||
| 24 | Chromium | 311 K [14] | ||
| 25 | Manganese | 100 K [14] | ||
| 26 | Iron | 1044 K [15] | ||
| 27 | Cobalt | 1390 K [15] | ||
| 28 | Nickel | 630 K [15] | ||
| 29 | Copper | 6 * 10−8 K [14] | ||
| 30 | Zinc | 0.85 K [13] | ||
| 31 | Gallium | 1.08 K [13] | ||
| 40 | Zirconium | 0.6 K [13] | ||
| 41 | Niobium | 9.25 K [13] | ||
| 42 | Molybdenum | 0.92 K [13] | ||
| 43 | Technetium | 8.2 K [13] | ||
| 44 | Ruthenium | 0.5 K [13] | ||
| 45 | Rhodium | 0.0003 K [13] | ||
| 46 | Palladium | 1.4 K [13] | ||
| 47 | Silver | 5.6 * 10−10 K [14] | ||
| 48 | Cadmium | 0.52 K [13] | ||
| 49 | Indium | 3.4 K [13] | ||
| 50 | Tin | 3.7 K [13] | ||
| 57 | Lanthanum | 6 K [13] | ||
| 58 | Cerium | 13 K [14] | ||
| 59 | Praseodymium | 25 K [14] | ||
| 60 | Neodymium | 19.9 K [14] | ||
| 62 | Samarium | 13.3 K [14] | ||
| 63 | Europium | 91 K [14] | ||
| 64 | Gadolinium | 293.4 K [15] | ||
| 65 | Terbium | 221 K [15] | 230 K [14] | |
| 66 | Dysprosium | 92.1 K [15] | 180.2 K [14] | |
| 67 | Holmium | 20 K [15] | 132.2 K [14] | |
| 68 | Erbium | 18.74 K [15] | 85.7 K [14] | |
| 69 | Thulium | 32 K [15] | 56 K [14] | |
| 71 | Lutetium | 0.1 K [13] | ||
| 72 | Hafnium | 0.38 K [13] | ||
| 73 | Tantalum | 4.4 K [13] | ||
| 74 | Tungsten | 0.01 K [13] | ||
| 75 | Rhenium | 1.7 K [13] | ||
| 76 | Osmium | 0.7 K [13] | ||
| 77 | Iridium | 0.1 K [13] | ||
| 80 | Mercury | 4.15 K [13] | ||
| 81 | Thallium | 2.4 K [13] | ||
| 82 | Lead | 7.2 K [13] | ||
| 90 | Thorium | 1.4 K [13] | ||
| 91 | Protactinium | 1.4 K [13] | ||
| 92 | Uranium | 1.3 K [13] | ||
| 95 | Americium | 1 K [13] |

Condensed matter physics is the field of physics that deals with the macroscopic and microscopic physical properties of matter, especially the solid and liquid phases that arise from electromagnetic forces between atoms and electrons. More generally, the subject deals with condensed phases of matter: systems of many constituents with strong interactions among them. More exotic condensed phases include the superconducting phase exhibited by certain materials at extremely low cryogenic temperatures, the ferromagnetic and antiferromagnetic phases of spins on crystal lattices of atoms, the Bose–Einstein condensates found in ultracold atomic systems, and liquid crystals. Condensed matter physicists seek to understand the behavior of these phases by experiments to measure various material properties, and by applying the physical laws of quantum mechanics, electromagnetism, statistical mechanics, and other physics theories to develop mathematical models and predict the properties of extremely large groups of atoms.

Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics. The word crystallography is derived from the Ancient Greek word κρύσταλλος, and γράφειν. In July 2012, the United Nations recognised the importance of the science of crystallography by proclaiming that 2014 the International Year of Crystallography.

Ferromagnetism is a property of certain materials that results in a significant, observable magnetic permeability, and in many cases, a significant magnetic coercivity, allowing the material to form a permanent magnet. Ferromagnetic materials are noticeably attracted to a magnet, which is a consequence of their substantial magnetic permeability.

Magnetism is the class of physical attributes that occur through a magnetic field, which allows objects to attract or repel each other. Because both electric currents and magnetic moments of elementary particles give rise to a magnetic field, magnetism is one of two aspects of electromagnetism.

Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, diamagnetic materials are repelled by magnetic fields and form induced magnetic fields in the direction opposite to that of the applied magnetic field. Paramagnetic materials include most chemical elements and some compounds; they have a relative magnetic permeability slightly greater than 1 and hence are attracted to magnetic fields. The magnetic moment induced by the applied field is linear in the field strength and rather weak. It typically requires a sensitive analytical balance to detect the effect and modern measurements on paramagnetic materials are often conducted with a SQUID magnetometer.

In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins pointing in opposite directions. This is, like ferromagnetism and ferrimagnetism, a manifestation of ordered magnetism. The phenomenon of antiferromagnetism was first introduced by Lev Landau in 1933.

In physics and materials science, the Curie temperature (TC), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Curie temperature is named after Pierre Curie, who showed that magnetism was lost at a critical temperature.
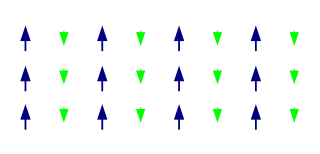
A ferrimagnetic material is a material that has populations of atoms with opposing magnetic moments, as in antiferromagnetism, but these moments are unequal in magnitude, so a spontaneous magnetization remains. This can for example occur when the populations consist of different atoms or ions (such as Fe2+ and Fe3+).
Carbon nanofoam is an allotrope of carbon discovered in 1997 by Andrei V. Rode and co-workers at the Australian National University in Canberra. It consists of a cluster-assembly of carbon atoms strung together in a loose three-dimensional web. The fractal-like bond structure consists of sp2 graphite-like clusters connected by sp3 bonds. The sp3 bonds are located mostly on the surface of the structure and make up 15% to 45% of the material, making its framework similar to diamond-like carbon films. The material is remarkably light, with a density of 2-10 x 10−3 g/cm3 (0.0012 lb/ft3) and is similar to an aerogel. Other remarkable physical properties include the large surface area of 300–400 m2/g. 1 US gallon of nanofoam weighs about 0.25 ounces (7.1 g).

A magnon is a quasiparticle, a collective excitation of the spin structure of an electron in a crystal lattice. In the equivalent wave picture of quantum mechanics, a magnon can be viewed as a quantized spin wave. Magnons carry a fixed amount of energy and lattice momentum, and are spin-1, indicating they obey boson behavior.

Rock magnetism is the study of the magnetic properties of rocks, sediments and soils. The field arose out of the need in paleomagnetism to understand how rocks record the Earth's magnetic field. This remanence is carried by minerals, particularly certain strongly magnetic minerals like magnetite. An understanding of remanence helps paleomagnetists to develop methods for measuring the ancient magnetic field and correct for effects like sediment compaction and metamorphism. Rock magnetic methods are used to get a more detailed picture of the source of the distinctive striped pattern in marine magnetic anomalies that provides important information on plate tectonics. They are also used to interpret terrestrial magnetic anomalies in magnetic surveys as well as the strong crustal magnetism on Mars.
Exchange bias or exchange anisotropy occurs in bilayers of magnetic materials where the hard magnetization behavior of an antiferromagnetic thin film causes a shift in the soft magnetization curve of a ferromagnetic film. The exchange bias phenomenon is of tremendous utility in magnetic recording, where it is used to pin the state of the readback heads of hard disk drives at exactly their point of maximum sensitivity; hence the term "bias."

Helimagnetism is a form of magnetic ordering where spins of neighbouring magnetic moments arrange themselves in a spiral or helical pattern, with a characteristic turn angle of somewhere between 0 and 180 degrees. It results from the competition between ferromagnetic and antiferromagnetic exchange interactions. It is possible to view ferromagnetism and antiferromagnetism as helimagnetic structures with characteristic turn angles of 0 and 180 degrees respectively. Helimagnetic order breaks spatial inversion symmetry, as it can be either left-handed or right-handed in nature.
In magnetism, a nanomagnet is a nanoscopic scale system that presents spontaneous magnetic order (magnetization) at zero applied magnetic field (remanence).
In solid state physics, a superstructure is some additional structure that is superimposed on a higher symmetry crystalline structure. A typical and important example is ferromagnetic ordering.
Magnetochemistry is concerned with the magnetic properties of chemical compounds. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons. Molecular compounds that contain one or more unpaired electrons are paramagnetic. The magnitude of the paramagnetism is expressed as an effective magnetic moment, μeff. For first-row transition metals the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula. In general, spin–orbit coupling causes μeff to deviate from the spin-only formula. For the heavier transition metals, lanthanides and actinides, spin–orbit coupling cannot be ignored. Exchange interaction can occur in clusters and infinite lattices, resulting in ferromagnetism, antiferromagnetism or ferrimagnetism depending on the relative orientations of the individual spins.
A domain wall is a term used in physics which can have similar meanings in magnetism, optics, or string theory. These phenomena can all be generically described as topological solitons which occur whenever a discrete symmetry is spontaneously broken.
In solid state physics, the magnetic space groups, or Shubnikov groups, are the symmetry groups which classify the symmetries of a crystal both in space, and in a two-valued property such as electron spin. To represent such a property, each lattice point is colored black or white, and in addition to the usual three-dimensional symmetry operations, there is a so-called "antisymmetry" operation which turns all black lattice points white and all white lattice points black. Thus, the magnetic space groups serve as an extension to the crystallographic space groups which describe spatial symmetry alone.

In condensed matter physics, altermagnetism is a type of persistent magnetic state in ideal crystals. Altermagnetic structures are collinear and crystal-symmetry compensated, resulting in zero net magnetisation. Unlike in an ordinary collinear antiferromagnet, another magnetic state with zero net magnetization, the electronic bands in an altermagnet are not Kramers degenerate, but instead depend on the wavevector in a spin-dependent way. Related to this feature, key experimental observations were published in 2024. It has been speculated that altermagnetism may have applications in the field of spintronics.
{{cite journal}}: CS1 maint: multiple names: authors list (link)