Related Research Articles

Fullerene chemistry is a field of organic chemistry devoted to the chemical properties of fullerenes. Research in this field is driven by the need to functionalize fullerenes and tune their properties. For example, fullerene is notoriously insoluble and adding a suitable group can enhance solubility. By adding a polymerizable group, a fullerene polymer can be obtained. Functionalized fullerenes are divided into two classes: exohedral fullerenes with substituents outside the cage and endohedral fullerenes with trapped molecules inside the cage.

The fluoronium ion is an inorganic cation with the chemical formula H
2F+
. It is one of the cations found in fluoroantimonic acid. The structure of the salt with the Sb
2F−
11 anion, has been determined. The fluoronium ion is isoelectronic with the water molecule and the azanide ion.
Dialkylbiaryl phosphine ligands are phosphine ligands that are used in homogeneous catalysis. They have proved useful in Buchwald-Hartwig amination and etherification reactions as well as Negishi cross-coupling, Suzuki-Miyaura cross-coupling, and related reactions. In addition to these Pd-based processes, their use has also been extended to transformations catalyzed by nickel, gold, silver, copper, rhodium, and ruthenium, among other transition metals.
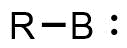
A borylene is the boron analogue of a carbene. The general structure is R-B: with R an organic moiety and B a boron atom with two unshared electrons. Borylenes are of academic interest in organoboron chemistry. A singlet ground state is predominant with boron having two vacant sp2 orbitals and one doubly occupied one. With just one additional substituent the boron is more electron deficient than the carbon atom in a carbene. For this reason stable borylenes are more uncommon than stable carbenes. Some borylenes such as boron monofluoride (BF) and boron monohydride (BH) the parent compound also known simply as borylene, have been detected in microwave spectroscopy and may exist in stars. Other borylenes exist as reactive intermediates and can only be inferred by chemical trapping.
A cycloparaphenylene is a molecule that consists of several benzene rings connected by covalent bonds in the para positions to form a hoop- or necklace-like structure. Its chemical formula is [C6H4]n or C
6nH
4n Such a molecule is usually denoted [n]CPP where n is the number of benzene rings.
Karl Wieghardt is a German inorganic chemist and emeritus director of the Max Planck Institute for Chemical Energy Conversion in Mülheim. He was active in the preparation and detailed characterization of models for iron and manganese metalloenzymes, metal complexes of noninnocent ligands, and magnetic interactions in polynuclear metal complexes.
Corinna S. Schindler is a Professor of Chemistry at the University of Michigan. She develops catalytic reactions with environmentally benign metals such as iron, towards the synthesis of biologically active small molecules. For her research in the development of new catalysts, Schindler has been honored with several early-career researcher awards including the David and Lucile Packard Foundation Fellowship in 2016, the Alfred P. Sloan Fellowship in 2017, and being named a member of the C&EN Talented 12 in 2017. Schindler has served on the Editorial Board of Organic and Bimolecular Chemistry since 2018.
Richard Dronskowski is a German chemist and physicist. He is a full professor at the RWTH Aachen University.
Hans Georg von Schnering was a German chemist and professor of inorganic chemistry at the University of Münster, honorary professor at the University of Stuttgart and director at the Max Planck Institute for Solid State Research.
Nathan C. Gianneschi is the Jacob & Rosaline Cohn Professor of Chemistry, Materials Science & Engineering, and Biomedical Engineering at Northwestern University and the Associate Director for the International Institute for Nanotechnology. Gianneschi's lab takes an interdisciplinary approach to nanomaterials research, with a focus on multifunctional materials for biomedical applications, programmed interactions with biomolecules and cells, and basic research into nanoscale materials design, synthesis and characterization.
Natalia B. Shustova is a Peter and Bonnie McCausland Professor of Chemistry at the University of South Carolina. She focuses on developing materials for sustainable energy conversion, metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and graphitic supramolecular structures.

Nontrigonal pnictogen compounds refer to tricoordinate trivalent pnictogen compounds that are not of typical trigonal pyramidal molecular geometry. By virtue of their geometric constraint, these compounds exhibit distinct electronic structures and reactivities, which bestow on them potential to provide unique nonmetal platforms for bond cleavage reactions.
Mark Stradiotto is a Canadian chemist. He is currently the Arthur B. McDonald Research Chair and the Alexander McLeod Professor of Chemistry in the Department of Chemistry at Dalhousie University.
Ellen Sletten is an American chemist who is the John McTague Career Development Chair at University of California, Los Angeles. Her research considers the use of physical organic chemistry for diagnostics and medical therapies.
The borosulfates are heteropoly anion compounds which have sulfate groups attached to boron atoms. Other possible terms are sulfatoborates or boron-sulfur oxides. The ratio of sulfate to borate reflects the degree of condensation. With [B(SO4)4]5- there is no condensation, each ion stands alone. In [B(SO4)3]3- the anions are linked into a chain, a chain of loops, or as [B2(SO4)6]6− in a cycle. Finally in [B(SO4)2]− the sulfate and borate tetrahedra are all linked into a two or three-dimensional network. These arrangements of oxygen around boron and sulfur can have forms resembling silicates. The first borosulfate to be discovered was K5[B(SO4)4] in 2012. Over 75 unique compounds are known.
The borophosphates are mixed anion compounds containing borate and phosphate anions, which may be joined together by a common oxygen atom. Compounds that contain water or hydroxy groups can also be included in the class of compounds.

Marinella Mazzanti is an Italian inorganic chemist specialized in coordination chemistry. She is a professor at EPFL and the head of the group of Coordination Chemistry at EPFL's School of Basic Sciences.

Jieping Zhu is an organic chemist specializing in natural product total synthesis and organometallics. He is a professor of chemistry at EPFL and the head of the Laboratory of Synthesis and Natural Products.
Karsten Meyer is a German inorganic chemist and Chair of Inorganic and General Chemistry at the Friedrich-Alexander University of Erlangen-Nürnberg (FAU). His research involves the coordination chemistry of transition metals as well as uranium coordination chemistry, small molecule activation with these coordination complexes, and the synthesis of new chelating ligands. He is the 2017 recipient of the Elhuyar-Goldschmidt Award of the Spanish Royal Society of Chemistry, the Ludwig-Mond Award of the Royal Society of Chemistry, and the L.A. Chugaev Commemorative Medal of the Russian Academy of Sciences, among other awards. He also serves as an Associate Editor of the journal Organometallics since 2014.
Harry Dorn is an American chemist and a professor of chemistry at Virginia Tech, since 1974. He was a professor of Radiology at Virginia Tech Carilion School of Medicine and a professor at Virginia Tech Fralin Biomedical Research Institute from 2012 to 2017.
References
- 1 2 "Marilyn Olmstead: My Life and Career in Chemistry | The Capitol Chemist" . Retrieved 2021-08-14.
- ↑ "Obituary: Marilyn Olmstead". cen.acs.org. Retrieved 2021-08-14.
- 1 2 3 "Remembering Professor Marilyn Olmstead". UC Davis Chemistry. 2020-10-08. Retrieved 2021-08-14.
- 1 2 "2014 ACS Fellows". cen.acs.org. Retrieved 2021-08-14.
- 1 2 "Three Alumni Named 2014 ACS Fellows". Department of Chemistry. 2014-07-22. Retrieved 2021-08-14.
- 1 2 "Women From Higher Education Named Fellows of American Chemical Society". Women In Academia Report. 2014-08-06. Retrieved 2021-08-14.
- 1 2 "Fellows of the American Crystallographic Association". www.amercrystalassn.org. Retrieved 2021-08-14.
- ↑ Mercado, Brandon Q.; Jiang, An; Yang, Hua; Wang, Zhimin; Jin, Hongxiao; Liu, Ziyang; Olmstead, Marilyn M.; Balch, Alan L. (2009). "Isolation and Structural Characterization of the Molecular Nanocapsule Sm2@D3d(822)-C104". Angewandte Chemie International Edition. 48 (48): 9114–9116. doi:10.1002/anie.200904662. ISSN 1521-3773.
- ↑ Pan, Changwang; Bao, Lipiao; Yu, Xianyong; Fang, Hongyun; Xie, Yunpeng; Akasaka, Takeshi; Lu, Xing (2018-02-27). "Facile Access to Y2C2n (2n = 92–130) and Crystallographic Characterization of Y2C2@C1(1660)-C108: A Giant Nanocapsule with a Linear Carbide Cluster". ACS Nano. 12 (2): 2065–2069. doi:10.1021/acsnano.8b00384. ISSN 1936-0851. PMID 29400943.
- ↑ Mercado, Brandon Q.; Olmstead, Marilyn M.; Beavers, Christine M.; Easterling, Michael L.; Stevenson, Steven; Mackey, Mary A.; Coumbe, Curtis E.; Phillips, Joshua D.; Phillips, J. Paige; Poblet, Josep M.; Balch, Alan L. (2009-12-15). "A seven atom cluster in a carbon cage, the crystallographically determined structure of Sc4(μ3-O)3@Ih-C80". Chemical Communications. 46 (2): 279–281. doi:10.1039/B918731F. ISSN 1364-548X.
- ↑ Zhang, Yang; Ghiassi, Kamran B.; Deng, Qingming; Samoylova, Nataliya A.; Olmstead, Marilyn M.; Balch, Alan L.; Popov, Alexey A. (2015). "Synthesis and Structure of LaSc2N@Cs(hept)-C80 with One Heptagon and Thirteen Pentagons". Angewandte Chemie International Edition. 54 (2): 495–499. doi:10.1002/anie.201409094. ISSN 1521-3773. PMID 25413484.
- ↑ Dahl, Jeremy E. P.; Moldowan, J. Michael; Peakman, Torren M.; Clardy, Jon C.; Lobkovsky, Emil; Olmstead, Marilyn M.; May, Paul W.; Davis, Tim J.; Steeds, John W.; Peters, Ken E.; Pepper, Andy (2003). "Isolation and Structural Proof of the Large Diamond Molecule, Cyclohexamantane (C26H30)". Angewandte Chemie International Edition. 42 (18): 2040–2044. doi:10.1002/anie.200250794. ISSN 1521-3773.
- ↑ Stevenson, S.; Rice, G.; Glass, T.; Harich, K.; Cromer, F.; Jordan, M. R.; Craft, J.; Hadju, E.; Bible, R.; Olmstead, M. M.; Maitra, K. (1999). "Small-bandgap endohedral metallofullerenes in high yield and purity". Nature. 401 (6748): 55–57. doi:10.1038/43415. ISSN 1476-4687. S2CID 4340875.
- ↑ Schreiner, Peter R.; Fokin, Andrey A.; Reisenauer, Hans Peter; Tkachenko, Boryslav A.; Vass, Elemér; Olmstead, Marilyn M.; Bläser, Dieter; Boese, Roland; Dahl, Jeremy E. P.; Carlson, Robert M. K. (2009-08-19). "[123]Tetramantane: Parent of a New Family of σ-Helicenes". Journal of the American Chemical Society. 131 (32): 11292–11293. doi:10.1021/ja904527g. ISSN 0002-7863. PMID 19722641.
- ↑ Olmstead, Marilyn M.; Lee, Hon Man; Duchamp, James C.; Stevenson, Steven; Marciu, Daniela; Dorn, Harry C.; Balch, Alan L. (2003). "Sc3N@C68: Folded Pentalene Coordination in an Endohedral Fullerene that Does Not Obey the Isolated Pentagon Rule". Angewandte Chemie International Edition. 42 (8): 900–903. doi:10.1002/anie.200390237. ISSN 1521-3773. PMID 12596171.
- ↑ Olmstead, Marilyn M.; Power, Philip P. (1986-07-01). "First structural characterization of a boron-centered radical: x-ray crystal structure of [Li(12-crown-4)2]+ [BMes3]-.bul". Journal of the American Chemical Society. 108 (14): 4235–4236. doi:10.1021/ja00274a071. ISSN 0002-7863.
- ↑ "Retired UCD chem professor killed while cycling on rural road". Davis Enterprise. 2020-10-01. Retrieved 2021-08-14.