T cells are one of the important types of white blood cells of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell receptor (TCR) on their cell surface.

A cytotoxic T cell (also known as TC, cytotoxic T lymphocyte, CTL, T-killer cell, cytolytic T cell, CD8+ T-cell or killer T cell) is a T lymphocyte (a type of white blood cell) that kills cancer cells, cells that are infected by intracellular pathogens (such as viruses or bacteria), or cells that are damaged in other ways.

The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the adaptive immune system. They aid the activity of other immune cells by releasing cytokines. They are considered essential in B cell antibody class switching, breaking cross-tolerance in dendritic cells, in the activation and growth of cytotoxic T cells, and in maximizing bactericidal activity of phagocytes such as macrophages and neutrophils. CD4+ cells are mature Th cells that express the surface protein CD4. Genetic variation in regulatory elements expressed by CD4+ cells determines susceptibility to a broad class of autoimmune diseases.

Cellular immunity, also known as cell-mediated immunity, is an immune response that does not rely on the production of antibodies. Rather, cell-mediated immunity is the activation of phagocytes, antigen-specific cytotoxic T-lymphocytes, and the release of various cytokines in response to an antigen.
The regulatory T cells (Tregs or Treg cells), formerly known as suppressor T cells, are a subpopulation of T cells that modulate the immune system, maintain tolerance to self-antigens, and prevent autoimmune disease. Treg cells are immunosuppressive and generally suppress or downregulate induction and proliferation of effector T cells. Treg cells express the biomarkers CD4, FOXP3, and CD25 and are thought to be derived from the same lineage as naïve CD4+ cells. Because effector T cells also express CD4 and CD25, Treg cells are very difficult to effectively discern from effector CD4+, making them difficult to study. Research has found that the cytokine transforming growth factor beta (TGF-β) is essential for Treg cells to differentiate from naïve CD4+ cells and is important in maintaining Treg cell homeostasis.

The adaptive immune system, also known as the acquired immune system, or specific immune system is a subsystem of the immune system that is composed of specialized, systemic cells and processes that eliminate pathogens or prevent their growth. The acquired immune system is one of the two main immunity strategies found in vertebrates.
Alloimmunity is an immune response to nonself antigens from members of the same species, which are called alloantigens or isoantigens. Two major types of alloantigens are blood group antigens and histocompatibility antigens. In alloimmunity, the body creates antibodies against the alloantigens, attacking transfused blood, allotransplanted tissue, and even the fetus in some cases. Alloimmune (isoimmune) response results in graft rejection, which is manifested as deterioration or complete loss of graft function. In contrast, autoimmunity is an immune response to the self's own antigens. Alloimmunization (isoimmunization) is the process of becoming alloimmune, that is, developing the relevant antibodies for the first time.
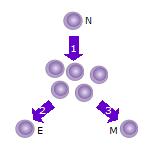
In immunology, a naive T cell (Th0 cell) is a T cell that has differentiated in the thymus, and successfully undergone the positive and negative processes of central selection in the thymus. Among these are the naive forms of helper T cells (CD4+) and cytotoxic T cells (CD8+). Any naive T cell is considered immature and, unlike activated or memory T cells, has not encountered its cognate antigen within the periphery. After this encounter, the naive T cell is considered a mature T cell.

Interleukin 21 (IL-21) is a protein that in humans is encoded by the IL21 gene.
Intraepithelial lymphocytes (IEL) are lymphocytes found in the epithelial layer of mammalian mucosal linings, such as the gastrointestinal (GI) tract and reproductive tract. However, unlike other T cells, IELs do not need priming. Upon encountering antigens, they immediately release cytokines and cause killing of infected target cells. In the GI tract, they are components of gut-associated lymphoid tissue (GALT).
In immunology, peripheral tolerance is the second branch of immunological tolerance, after central tolerance. It takes place in the immune periphery. Its main purpose is to ensure that self-reactive T and B cells which escaped central tolerance do not cause autoimmune disease. Peripheral tolerance prevents immune response to harmless food antigens and allergens, too.

CD69 is a human transmembrane C-Type lectin protein encoded by the CD69 gene. It is an early activation marker that is expressed in hematopoietic stem cells, T cells, and many other cell types in the immune system. It is also implicated in T cell differentiation as well as lymphocyte retention in lymphoid organs.
Gamma delta T cells are T cells that have a γδ T-cell receptor (TCR) on their surface. Most T cells are αβ T cells with TCR composed of two glycoprotein chains called α (alpha) and β (beta) TCR chains. In contrast, γδ T cells have a TCR that is made up of one γ (gamma) chain and one δ (delta) chain. This group of T cells is usually less common than αβ T cells. Their highest abundance is in the gut mucosa, within a population of lymphocytes known as intraepithelial lymphocytes (IELs).

Lymphocyte-activation gene 3, also known as LAG-3, is a protein which in humans is encoded by the LAG3 gene. LAG3, which was discovered in 1990 and was designated CD223 after the Seventh Human Leucocyte Differentiation Antigen Workshop in 2000, is a cell surface molecule with diverse biological effects on T cell function but overall has an immune inhibitory effect. It is an immune checkpoint receptor and as such is the target of various drug development programs by pharmaceutical companies seeking to develop new treatments for cancer and autoimmune disorders. In soluble form it is also being developed as a cancer drug in its own right.
T helper 3 cells (Th3) are a subset of T lymphocytes with immunoregulary and immunosuppressive functions, that can be induced by administration of foreign oral antigen. Th3 cells act mainly through the secretion of anti-inflammatory cytokine transforming growth factor beta (TGF-β). Th3 have been described both in mice and human as CD4+FOXP3− regulatory T cells. Th3 cells were first described in research focusing on oral tolerance in the experimental autoimmune encephalitis (EAE) mouse model and later described as CD4+CD25−FOXP3−LAP+ cells, that can be induced in the gut by oral antigen through T cell receptor (TCR) signalling.
Natural killer T (NKT) cells are a heterogeneous group of T cells that share properties of both T cells and natural killer cells. Many of these cells recognize the non-polymorphic CD1d molecule, an antigen-presenting molecule that binds self and foreign lipids and glycolipids. They constitute only approximately 1% of all peripheral blood T cells. Natural killer T cells should neither be confused with natural killer cells nor killer T cells.
Mucosal-associated invariant T cells make up a subset of T cells in the immune system that display innate, effector-like qualities. In humans, MAIT cells are found in the blood, liver, lungs, and mucosa, defending against microbial activity and infection. The MHC class I-like protein, MR1, is responsible for presenting bacterially-produced vitamin B2 and B9 metabolites to MAIT cells. After the presentation of foreign antigen by MR1, MAIT cells secrete pro-inflammatory cytokines and are capable of lysing bacterially-infected cells. MAIT cells can also be activated through MR1-independent signaling. In addition to possessing innate-like functions, this T cell subset supports the adaptive immune response and has a memory-like phenotype. Furthermore, MAIT cells are thought to play a role in autoimmune diseases, such as multiple sclerosis, arthritis and inflammatory bowel disease, although definitive evidence is yet to be published.
Tissue-resident memory T cells or TRM cells represent a subset of a long-lived memory T cells that occupies epithelial, mucosal and other tissues without recirculating. TRM cells are transcriptionally, phenotypically and functionally distinct from central memory (TCM) and effector memory (TEM) T cells which recirculate between blood, the T cell zones of secondary lymphoid organ, lymph and nonlymphoid tissues. Moreover, TRM cells themself represent a diverse populations because of the specializations for the resident tissues. The main role of TRM cells is to provide superior protection against infection in extralymphoid tissues.
Virtual memory T cells (TVM) are a subtype of T lymphocytes. These are cells that have a memory phenotype but have not been exposed to a foreign antigen. They are classified as memory cells but do not have an obvious memory function. They were first observed and described in 2009. The name comes from a computerized "virtual memory" that describes a working memory based on an alternative use of an existing space.
A T memory stem cell (TSCM) is a type of long-lived memory T cell with the ability to reconstitute the full diversity of memory and effector T cell subpopulations as well as to maintain their own pool through self-renewal. TSCM represent an intermediate subset between naïve (Tn) and central memory (Tcm) T cells, expressing both naïve T cells markers, such as CD45RA+, CD45RO-, high levels of CD27, CD28, IL-7Rα (CD127), CD62L, and C-C chemokine receptor 7 (CCR7), as well as markers of memory T cells, such as CD95, CD122 (IL-2Rβ), CXCR3, LFA-1. These cells represent a small fraction of circulating T cells, approximately 2-3%. Like naïve T cells, TSCM cells are found more abundantly in lymph nodes than in the spleen or bone marrow; but in contrast to naïve T cells, TSCM cells are clonally expanded. Similarly to memory T cells, TSCM are able to rapidly proliferate and secrete pro-inflammatory cytokines in response to antigen re-exposure, but show higher proliferation potential compared with Tcm cells; their homeostatic turnover is also dependent on IL-7 and IL-15.